
Key Points
- Global debut: JTI’s next-generation heated-tobacco device Ploom AURA has officially launched in Switzerland, signaling the start of its worldwide rollout.
- Technology upgrades: Equipped with the SMART HEATFLOW™ intelligent heating system and four Heat Select modes.
- Switzerland’s strategic weight: JTI is headquartered in Geneva; Dagmersellen is one of four global production bases for EVO filter mouthpieces, demonstrating long-term investment in Switzerland.
- Large investment: JTI plans to invest about JPY 650 billion (approximately USD 4.5 billion) from 2025–2027 to accelerate its Reduced-Risk Products (RRP) portfolio, with Ploom AURA as a core project.
2Firsts, September 2, 2025 — 2Firsts has learned from JTI’s official website that Japan Tobacco International (JTI) has officially launched its next-generation heated-tobacco device, Ploom AURA, in Switzerland, marking the beginning of a global expansion. Following its release in Japan, Ploom AURA will gradually enter additional markets.

According to JTI, Ploom AURA features a proprietary SMART HEATFLOW™ intelligent heating system that optimizes temperature control to deliver more consistent tobacco flavor while avoiding the unpleasant odors and health risks associated with combustion. The device also includes the Heat Select system, offering four heating modes so users can tailor their experience, moving beyond the traditional “single-mode” limitation.
2Firsts has also published a detailed analysis of the product (Related reading: “Product| Japan Tobacco Launches Ploom AURA and EVO Tobacco Device). See the image below for details.
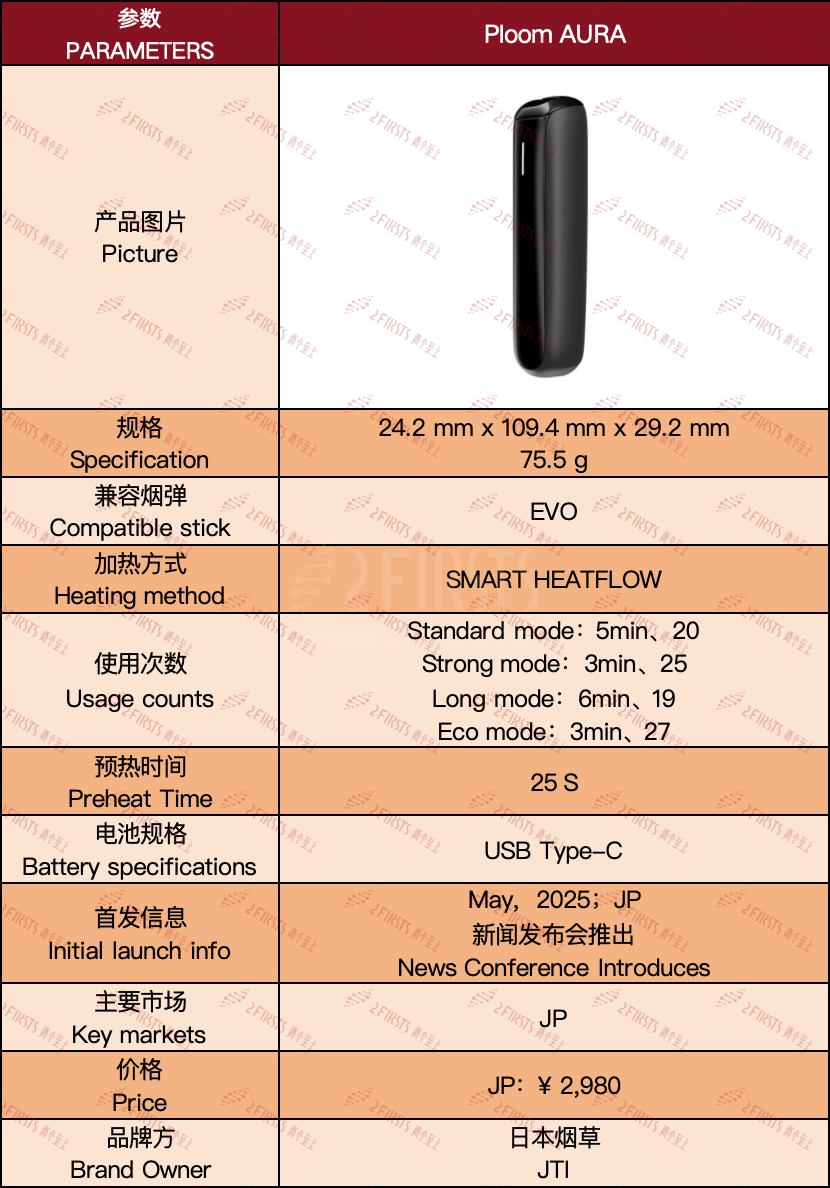
For more on Ploom AURA’s R&D background, technical principles, and global strategy, see 2Firsts’ in-depth conversation with Nick Geens, JTI’s Vice President for RRP Strategy & Planning: 2Firsts Exclusive Interview | JTI Launches New-Generation Ploom AURA, Focuses on Enhanced Flavor and Customization
JTI emphasized that the Swiss launch is not only a product release but also a key step in its global strategic deployment. Switzerland plays a central role in JTI’s global operations: the company’s headquarters is in Geneva, and Dagmersellen in the canton of Lucerne houses both the Swiss market’s marketing team and an advanced manufacturing site—one of only four global facilities producing EVO filter mouthpieces.
JTI disclosed plans to invest JPY 650 billion (USD 4.483 billion) between 2025 and 2027 in innovative Reduced-Risk Products (RRP), with Ploom AURA’s internationalization among the top priorities. As of July 31, 2025, JTI held a 13.6% market share in Japan’s HTS segment, reflecting the overall performance of its heated tobacco portfolio, including Ploom AURA and previous-generation devices.
The Swiss launch underscores JTI’s long-term commitment to meeting the diverse needs of adult tobacco consumers through technological innovation.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







