Special statement:
This article is only for internal industry communication and does not make any recommendations for brands or products.
The pictures displayed in this article are solely used to describe the facts and are not intended as advertisements for any products.
This article is off-limits to minors.
Key Points:
New Product Announcement: The UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product notification database shows that brands such as SKE, ELFBAR, and IVG have all announced new products, which have received approval for sale in the UK market.
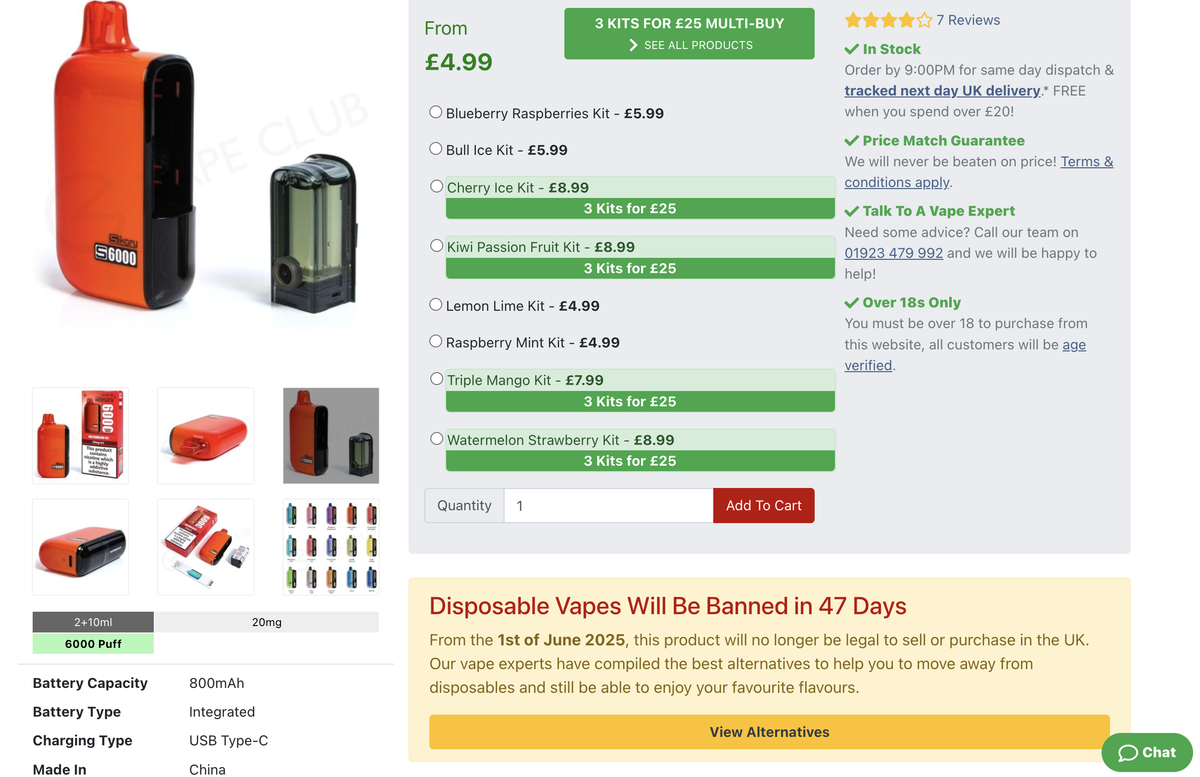
e-cigarette flavor update SKU: SKE SIKARY S6000 disposable e-cigarette has launched 10 new flavors, but due to a ban in the UK, they will no longer be legally sold starting from June 1, 2025.
e-cigarette device new SKU: Heaven Gifts' LOST MARY in collaboration with HAWCOS has released the HAWCOS×LOST MARY PRO MAX 7000 Device, while ELFBAR and ELFLIQ have released ELFBAR ELFA TURBO Aurora.
Refillable e-cigarette new SKU: IVG has launched the IVG Smart Max refillable e-cigarette, containing six e-liquid flavors with a nicotine content of 20MG each.
Recently, through inquiries on the MHRA e-cigarette product notification database, brands such as SIKARY, ELFBAR, and IVG have announced new SKUs for public viewing. Once these new SKUs complete the notification process with the MHRA, it indicates that they have received approval for sale and will officially enter the UK market for distribution.
2Firsts has compiled and analyzed the information from the Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product alert database for the second week of April in the UK. The new SKUs of various e-cigarette brands encompass a wide range of e-cigarette types. Below is a detailed overview of the information disclosed.
E-cigarette flavors updated in SKU

The e-cigarette brand SKE SIKARY S6000 recently announced 10 new flavors, all with a nicotine concentration of 20MG/ML.
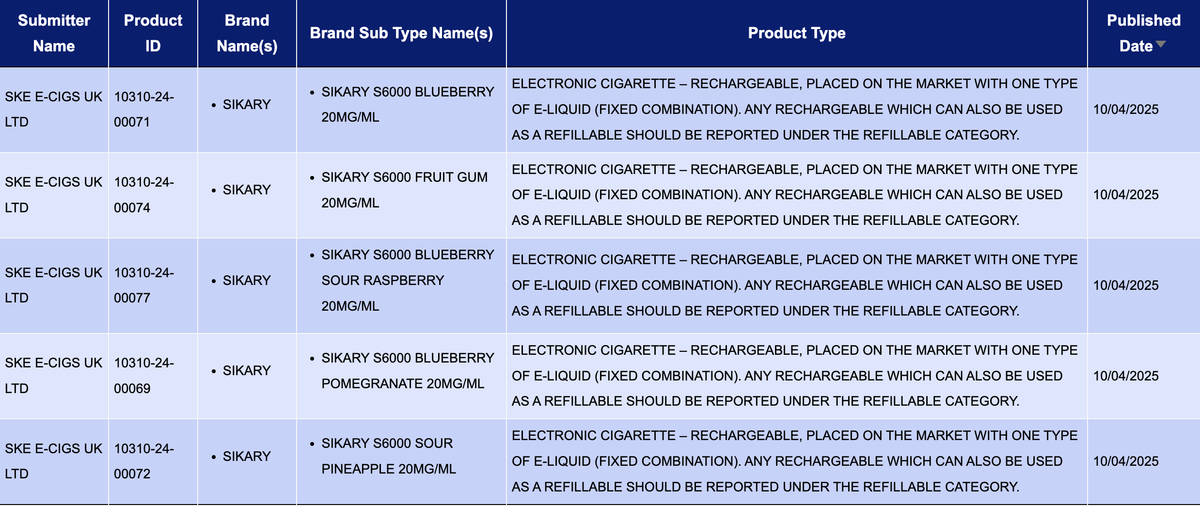
It is worth noting that 2Firsts found that the SKE SIKARY S6000 e-cigarette, currently being sold online by retailers, is no longer legal to purchase or sell in the UK starting June 1, 2025 due to its designation as a "disposable e-cigarette." However, on the MHRA official website, the SKE SIKARY S6000 is marked as:
E-cigarettes are sold as rechargeable devices paired with a specific type of e-liquid that cannot be changed by the user. Any rechargeable device that can be filled with e-liquid should be classified as a "refillable device".
Heaven Gifts introduces new e-cigarette devices
Recently, a total of 4 companies have added new SKUs for their new e-cigarette devices.
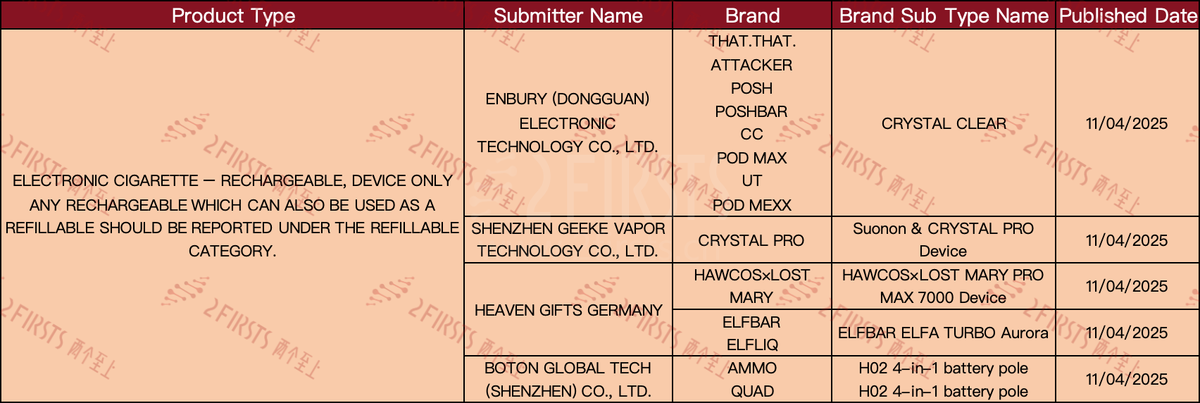
Heaven Gifts' brand Lost Mary has partnered with Hawcos to launch the Hawcos x Lost Mary Pro Max 7000 Device; while Elfbar brand Elfliq has introduced the new Elfbar Elfa Turbo Aurora.

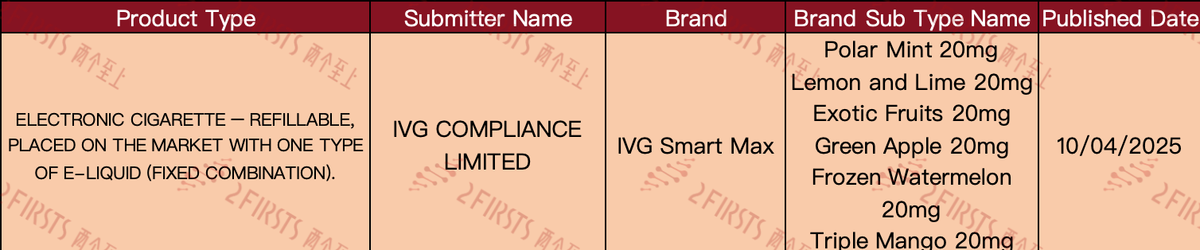
New IVG open-style product
Recently, e-cigarette brand IVG has announced a new refillable e-cigarette model called IVG Smart Max. The product comes in six e-liquid flavors: Polar Mint, Lemon and Lime, Exotic Fruits, Green Apple, Frozen Watermelon, and Triple Mango, with a nicotine content of 20MG.

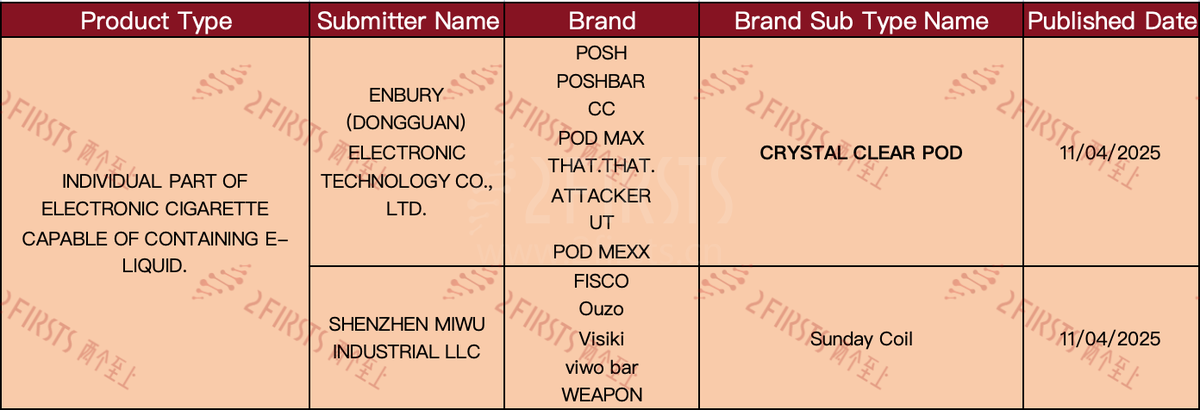
Other e-cigarette components
Refillable e-cigarette devices.

e-cigarette kit:

Components of an e-cigarette capable of holding e-liquid:
The Medicines and Healthcare products Regulatory Agency (MHRA) is the UK government agency responsible for regulating pharmaceuticals, medical devices, blood products, and nicotine-containing e-cigarette products.
If you have any unique insights or information, feel free to contact us at info@2firsts.com or reach out to 2Firsts CEO Alan Zhao on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







