
Special announcement:
This article is for internal industry communication only and does not make any recommendations for brands or products.
The images displayed in this article are solely used to describe facts and are not meant to serve as advertisements for any products.
This article is prohibited for minors to access.
NEXA, recently launched a new e-cigarette model, NEXA ULTRA InvisaCloud Edition. Vape.hk, a professional e-cigarette website, describes it as the "world's first smokeless e-cigarette device.
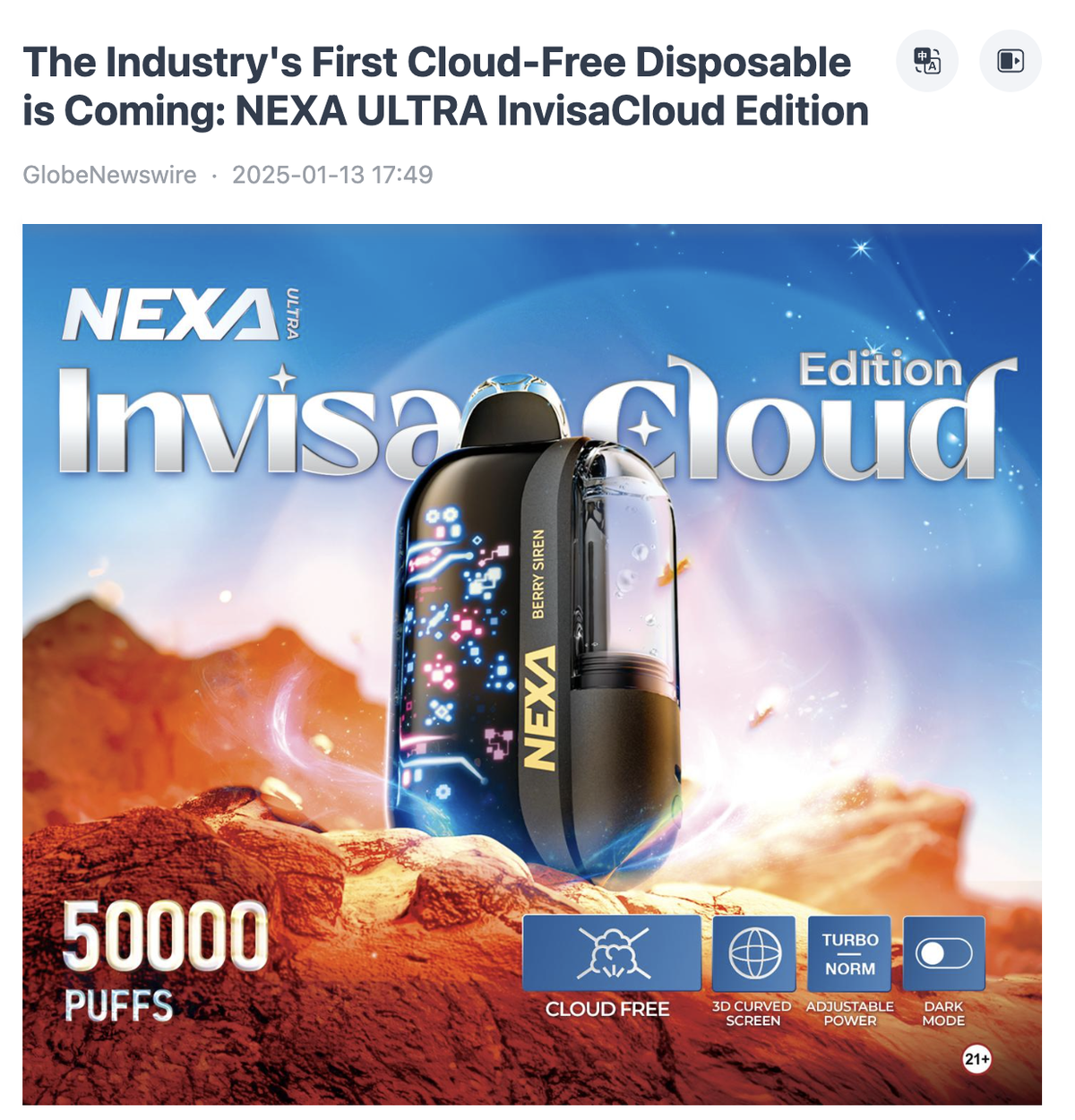
Specifically, the specifications of the NEXA ULTRA InvisaCloud Edition are shown in the table below.

According to a detailed introduction by vape.hk, the core highlight of the NEXA ULTRA InvisaCloud Edition is its innovative "smokeless" feature. The product offers users two unique inhalation modes: "light draw" and "deep draw". In the light draw mode, users can enjoy a rich smoke effect, while in the deep draw mode, they can experience a completely smokeless inhalation experience. It is reported that this smokeless technology optimizes the user's smoking experience by precisely controlling the heating temperature to avoid combustion.

The information released on the Yahoo website introduces the NEXA ULTRA InvisaCloud Edition as an upgraded version that retains the brand's previous NEXA ULTRA design.
According to reports, in October 2024, the NEXA brand launched the NEXA Ultra 50K disposable e-cigarette product, which at the time had the largest puff count of 50,000 in the American market. This product stood out for its large number of puffs and was known as the e-cigarette with the largest puff count in the American market at that time. (For more details, please read "Product | NEXA launches 50,000 puff e-cigarette Ultra 50K with 3D curved screen debuts in the United States")
Based on the information available, the new NEXA ULTRA InvisaCloud Edition not only increases the number of suction ports in turbo mode from 30,000 to 40,000, but also upgrades its e-liquid tank to a fully transparent design.

Currently, detailed corporate information about the NEXA brand and its products cannot be found on the Aiqicha website. However, contact information for the NEXA ULTRA InvisaCloud Edition product is listed as being located in Kowloon, Hong Kong on information websites such as Yahoo and Reportify.
2Firsts will continue to monitor the performance and market trends of e-cigarette products globally, providing accurate and authoritative industry information to the public.
2FIRSTS column is dedicated to providing readers with the latest product information in the tobacco field. We aim to collect and share first-hand information within the industry, and welcome readers to submit articles and share the latest products in the e-cigarette industry with us.
If you have any unique insights or information, please feel free to contact us at info@2firsts.com at any time.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








