
Special statement:
This article is for internal industry communication only, and does not make any recommendations for brands or products.
The images presented in this article are solely for descriptive purposes and are not intended as advertising for any product.
This article is prohibited for minors to access.
Product highlights:
Compliance Certification: The OXVA Slim Stick has been publicly listed in the Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product notification database in the United Kingdom.
Product Type: The OXVA Slim Stick is a pod system that uses pre-filled 2 milliliter pods that cannot be refilled.
Key parameters: The device has a battery capacity of 1500mAh, providing approximately 600 puffs per charge. The pod is available in 11 different flavors for users to choose from.
Market information: Available on the UK retailer's website, the package is priced at around 9.99 pounds, while the pod can be purchased separately for approximately 4.99 pounds (two pods per box).
The "UK E-cigarette Approved List Update" released by 2Firsts shows that the OXVA Slim Stick e-cigarette device and its pods, under the brand OXVA owned by Shenzhen Future Tech CO.,Limited completed their notification in the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product notification database on August 7. Recently, 2Firsts noted that the OXVA Slim Stick Pod kit has been listed on e-cigarette distributor websites in the UK, with the product priced at approximately £9.99.
OXVA launches pod-based e-cigarette with 2ml pod capacity
Firsts has compiled and organized the parameter information for the OXVA Slim Stick e-cigarette, which is detailed in the image below.
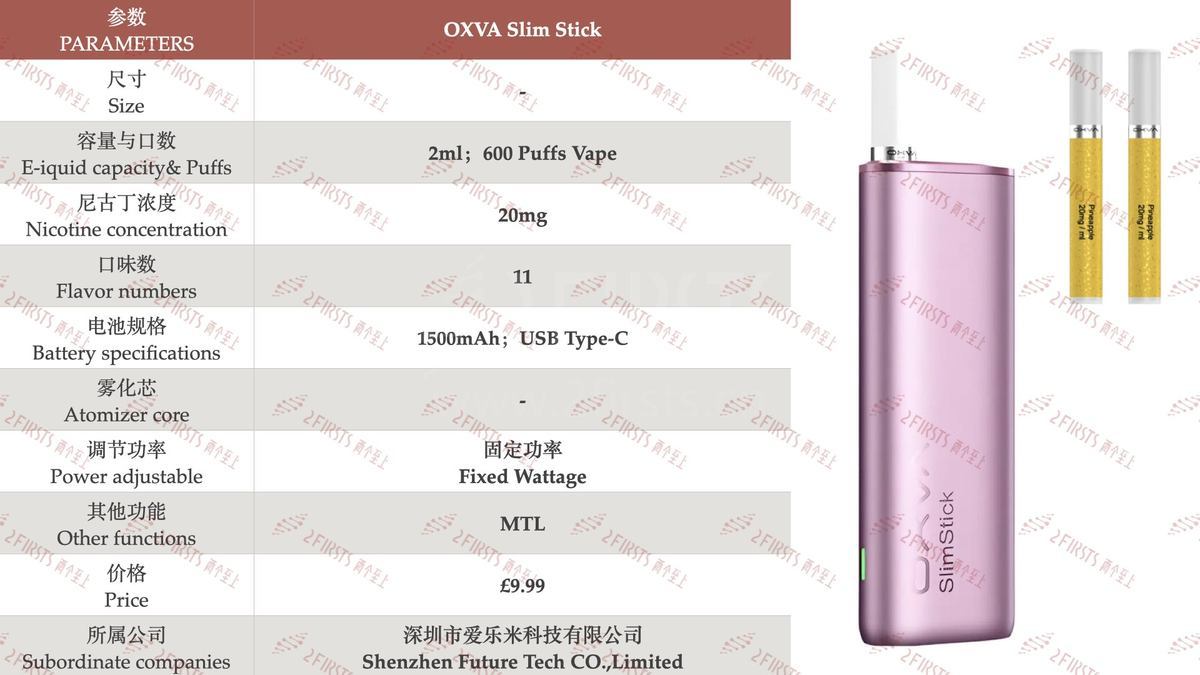
Unlike previous products released by the OXVA brand, the OXVA Slim Stick is a pod system, rather than an open system. The distributor website Vape Sourcing describes it as "It comes with 2ml prefilled pods, which are not refillable.

The pod capacity of the OXVA Slim Stick is 2 milliliters, and the brand claims it can provide 600 puffs per fill. The product is equipped with a 1500mAh battery, which has a larger capacity.
In addition, each package of an OXVA Slim Stick includes one OXVA Slim Stick device and two pods, allowing users to choose their own flavors. Currently, the distributor's website shows that OXVA Slim Stick pods are available in a total of 11 flavors, including tobacco, cola ice, and various fruit flavors.
The appearance design is closer to the HTP device
In July of this year, 2Firsts noted that several e-cigarette brands have started introducing magnetic suction open devices, which are gradually moving closer to Heat Tobacco Products (HTP) in terms of design, such as the IQOS ILUMA.
In a recent observation, it was found that the OXVA Slim Stick and the HNB product glo Hyper share a high degree of similarity in design. Both feature a slim and vertical body with a top-inserted consumable (tobacco stick/pod) structure, as well as a metallic shell with color schemes that give off a clean and tech-savvy visual impression. Additionally, the design of the LED indicator light on the side is also similar between the two products.
However, in terms of functionality, the two belong to different categories. The glo Hyper is a HTP device that uses tobacco sticks for heating, while the OXVA Slim Stick is an electronic vaporizer (Vape) that uses pods filled with e-liquid.
This similarity in appearance and structural logic reflects a new trend in e-cigarette manufacturers' product design, which is to draw inspiration from the appearance elements and user interaction methods of HNB products to attract a wider range of consumers.

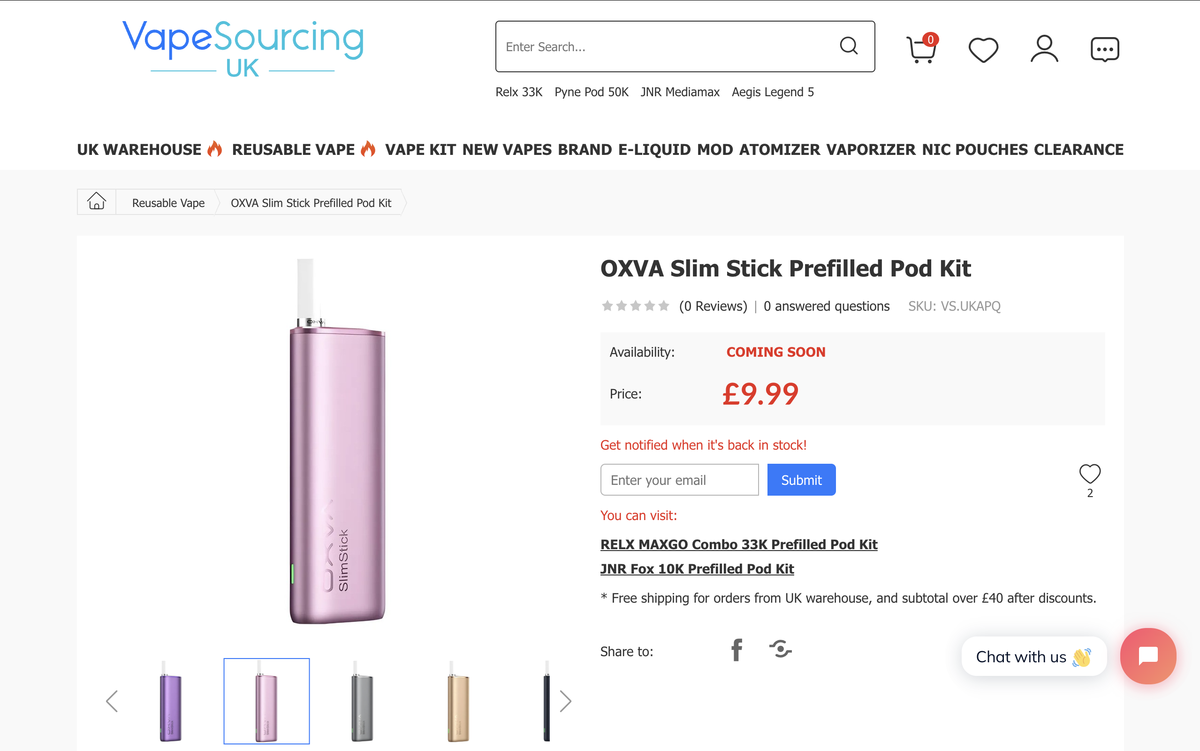
It is being sold online in the UK at a price of £9.99
The OXVA Slim Stick e-cigarette kit has landed on UK e-cigarette distributor websites such as Vape Club and Vape Sourcing. The product status is listed as "COMING SOON," with a price of approximately £9.99.
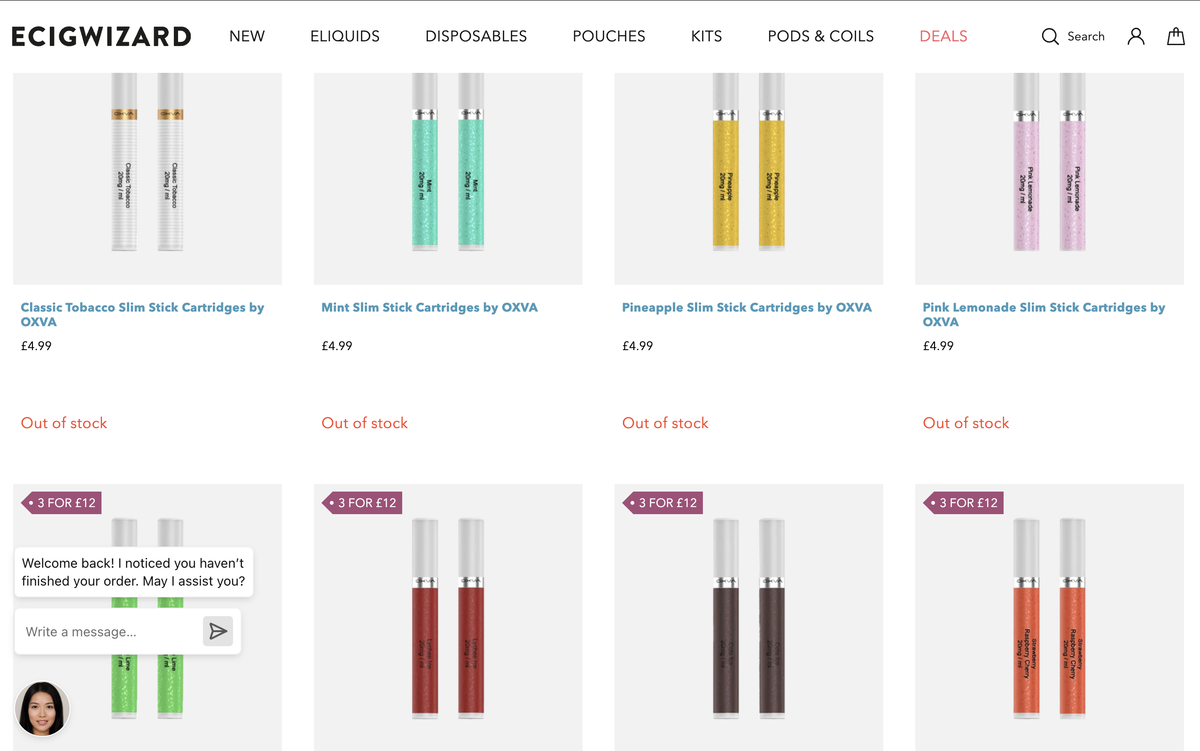
The pods for the OXVA Slim Stick have also been launched on the ECIGWIZARD website. Each pod (a box containing two pods) is priced at 4.99 pounds, while a pack of three pods is priced at 12 pounds.
As of now, the product has not been officially launched on the OXVA website.
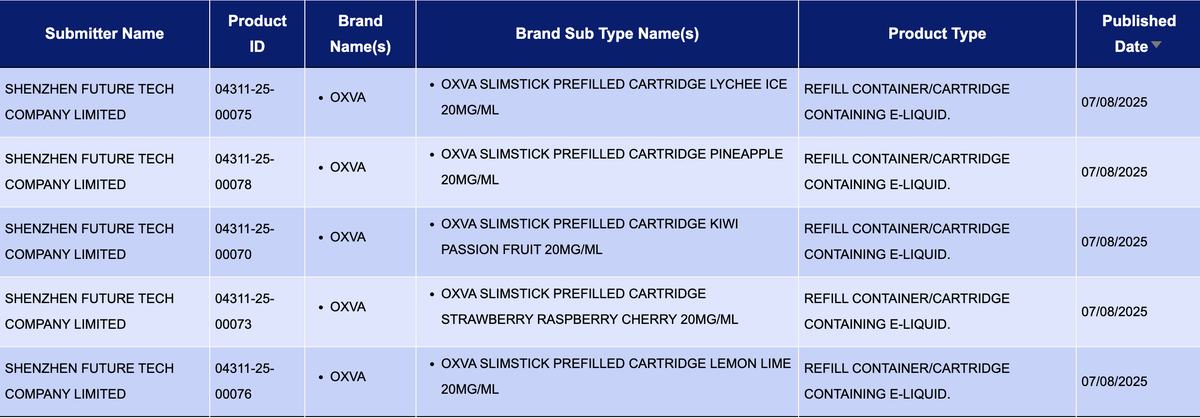
2Firsts conducted a systematic monitoring and organization of the announcements in the e-cigarette product database of the UK Medicines and Healthcare Products Regulatory Agency (MHRA). It was found that the "OXVA SlimStick Battery" and "OXVA SlimStick Prefilled Cartridge" series pods from the brand under Shenzhen Future Tech CO.,Limited were both officially announced on August 7th, with a total of 11 flavors listed for the products.
As a leading global NGP media and think tank, 2Firsts is committed to providing the latest product and technology information and insights to practitioners around the world in categories such as e-cigarettes, heated tobacco products, and modern oral products. We strive to drive technological change and innovation in NGP products globally, ultimately bringing safer products and lifestyles to tobacco consumers worldwide.
With information sources covering the supply chain in China and global markets, 2Firsts' product reporting has become one of the most influential platforms for new product and technology launches worldwide.
Contact 2Firsts for the following services:
1. Providing leads on new products and technologies;
2. Offering comments on products and technologies;
3. Seeking media coverage for your products;
4. Researching sales channels for your products;...
Contact information: Email: info@2firsts.com.
Connect with 2Firsts CEO Alan Zhao on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







