
Special Notice:
1. This article is intended for internal industry communication only and does not endorse any brands or products.
2. The images shown in this article are for factual description purposes only and not intended as product advertisements.
3. This article is not suitable for minors to access.
Key points:
During the period from August 2nd to August 10th, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK published a total of 329 e-cigarette SKUs in their e-cigarette product notification database, including brands such as ELFBAR, SKE, OXVA, and INNOKIN.
INNOKIN and OXVA's new e-cigarette devices have been officially unveiled and are now available on the e-cigarette distributor website in the UK. Both EasyJoy and the British brand FEOBA have revealed multiple refillable devices.
Among all the publicly listed SKUs, pod-type products account for the vast majority, with a total of 319. Brands such as SKE, OXVA, and ELFBAR have a large number of new pod products listed, with nicotine concentrations mainly concentrated at 10mg/ml and 20mg/ml.
Recently, 2Firsts conducted systematic monitoring and organization of the public content of the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product notification database from August 2nd to August 10th, 2025. During this period, MHRA released a total of 329 SKUs, including brands such as ELFBAR, OXVA, INNOKIN, SKE, and VAPES BARS.
According to the MHRA regulatory process, a product being published in the notification database means that the SKU has passed compliance review and obtained legal sales permission in the UK market. The following are the major brands, product types, and preliminary market trend analysis included in this notification update:
INNOKIN and OXVA have launched new e-cigarette devices, with the new brand NEXEL also releasing new products
In the product category “Electronic cigarette – Rechargeable, device only” (Any rechargeable which can also be used as a refillable should be reported under the refillable category), a total of 2 SKUs were updated from August 2 to August 10.

Under the brand INNOKIN of Shenzhen Xinyikang Technology Co., Ltd., the MVP 60K Device has been published. According to distributor websites, the product offers more than nine flavor options, is powered by a built-in 1000mAh battery, and is currently in the “COMING SOON” stage.

Under the brand OXVA of Shenzhen Future Tech CO., Ltd., the OXVA SlimStick Battery has been published. This product comes with a 2ml pre-filled pod, is powered by a built-in 1500mAh battery, and is currently in the “COMING SOON” stage.

In the product category “Electronic cigarette – Refillable, placed on the market with one type of e-liquid (fixed combination)”, a total of 2 SKUs were updated from August 2 to August 10.

Under the brand NEXEL of NEXGEN TECHNOLOGY HK LIMITED, the following products have been published:
AERO Refillable Pod – Fruit Gum 20mg/ml
AERO Refillable Pod – Fruit Fusion 20mg/ml
A search by 2FIRSTS on Alibaba Cloud’s trademark database shows that the NEXEL trademark under Class 34 (tobacco and smoking articles) was applied for registration by Xinyidai Technology Co., Ltd. in March 2025. The company has also applied for the AERO RUSH trademark under Class 34.
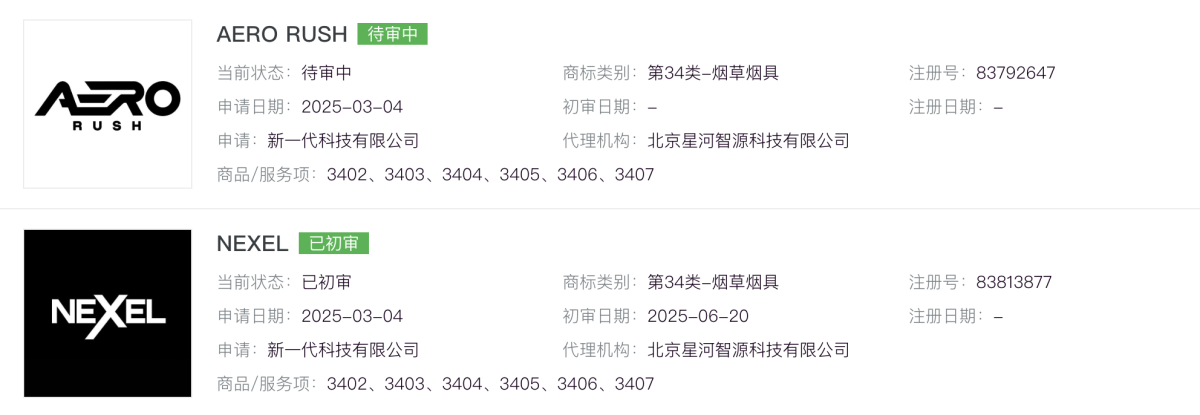
The first appearance of the NEXEL brand in the MHRA e-cigarette notification system was on June 25, 2025, indicating that NEXEL is a newcomer in the UK compliant e-cigarette market.
Eigate and UK brand FEOBA launch new open-system devices
In the product category “Electronic cigarette – Refillable, device only”, a total of 4 SKUs were updated from August 2 to August 10.
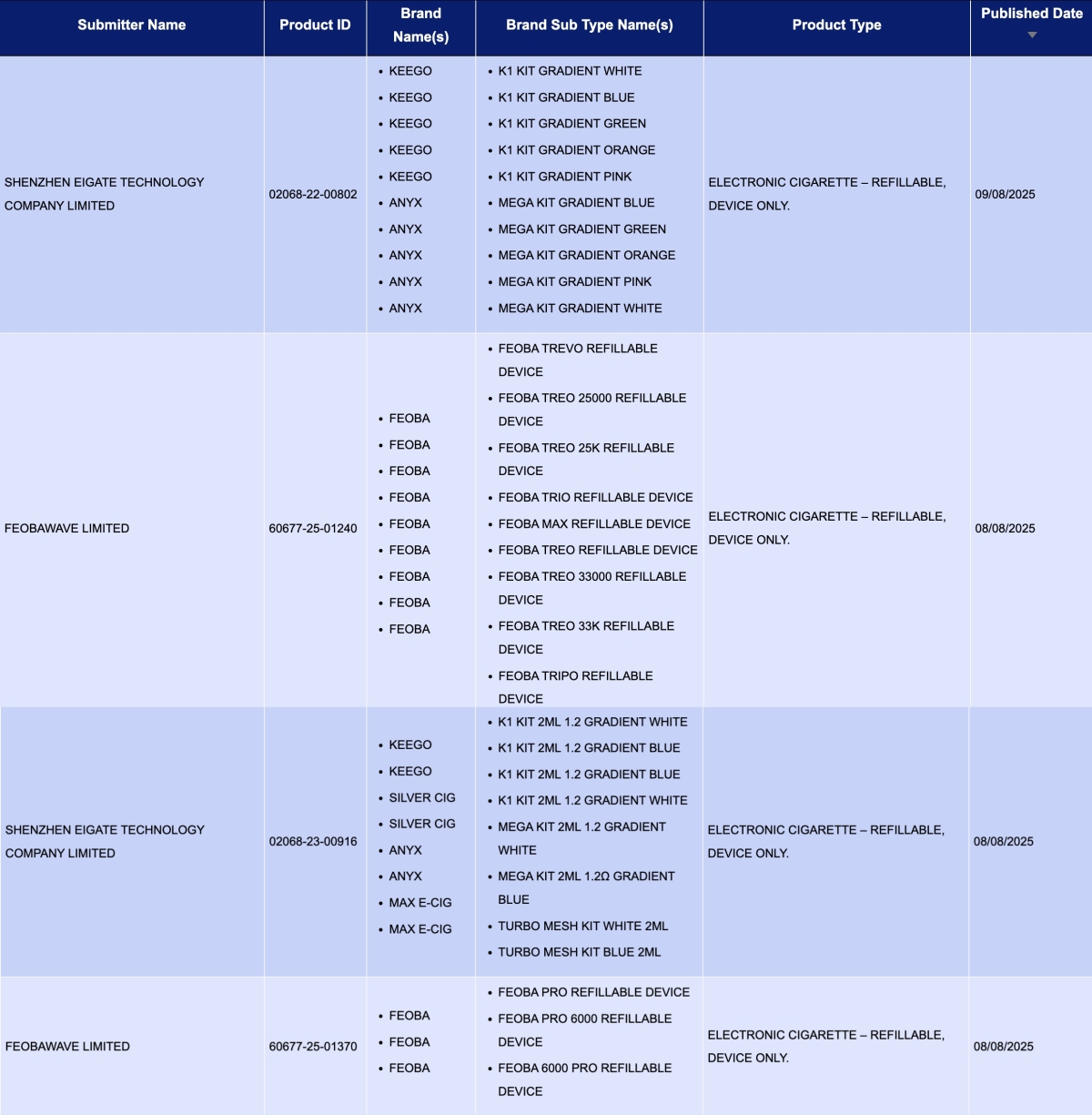
Under the brands of Shenzhen Eigate Technology Co.,Ltd. —KEEGO, ANYX, SILVER CIG, and MAX E-CIG—the following products were published:
K1 Kit Gradient (KEEGO)
K1 Kit 2ml 1.2 Gradient (KEEGO)
MEGA Kit Gradient (ANYX)
MEGA Kit 2ml (ANYX)
K1 Kit 2ml 1.2 Gradient (SILVER CIG)
Turbo Mesh Kit (MAX E-CIG)
Under FEOBAWAVE LIMITED’s brand FEOBA, the following products were published:
TREO Series (same SKU):
FEOBA TREVO Refillable Device
FEOBA TREO Refillable Device
FEOBA TREO 25000 Refillable Device
FEOBA TREO 33000 Refillable Device
Feoba Pro Series (same SKU):
Feoba Pro Refillable Device
Feoba Pro 6000 Refillable Device
Feoba 6000 Pro Refillable Device
319 New Pod SKUs Updated — Includes ELFBAR, SKE, OXVA and Other Brands
In the product category “Refill container/cartridge containing e-liquid”, a total of 319 SKUs were updated from August 2 to August 10, involving brands such as FEOBA, SKE, OXVA, ELFBAR, VAPES BARS.
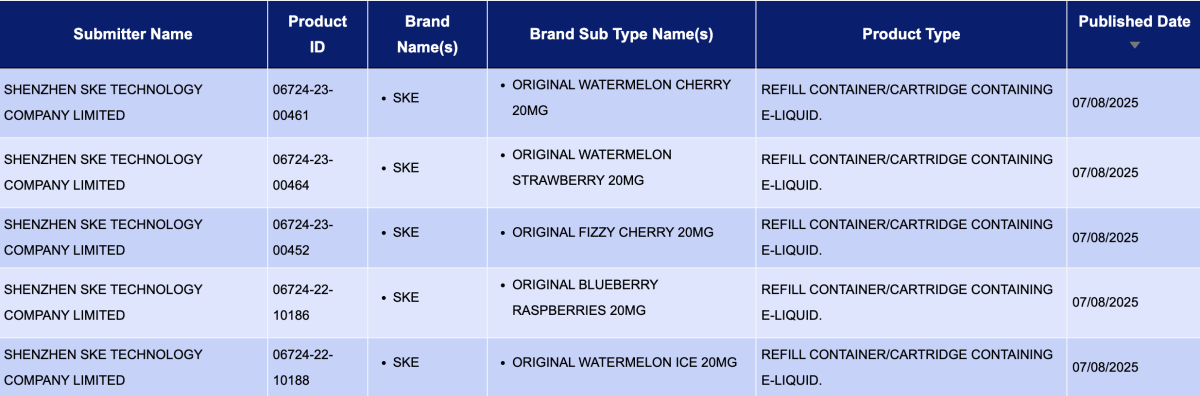
Under the brand SKE of Shenzhen SKE Technology Co., Ltd., 66 newly published SKUs have nicotine concentrations mainly at 10mg/ml and 20mg/ml.
Under the brand OXVA of Shenzhen Future Tech CO.,Limited, 11 newly published SKUs have nicotine concentrations mainly at 20mg/ml, suitable for the OXVA SlimStick Prefilled Cartridge e-cigarette.
Under the brand ELFBAR of IMiracles (Shenzhen) Technology Co., Ltd., 92 newly published SKUs have nicotine concentrations mainly at 20mg/ml, suitable for ELFBAR DUAL10K, ELFBAR 4IN1 ULTRA50, ELFBAR DUAL REFILLABLE POD e-cigarettes.
In the product category “Individual part of electronic cigarette capable of containing e-liquid”, a total of 2 SKUs were updated from August 2 to August 10.
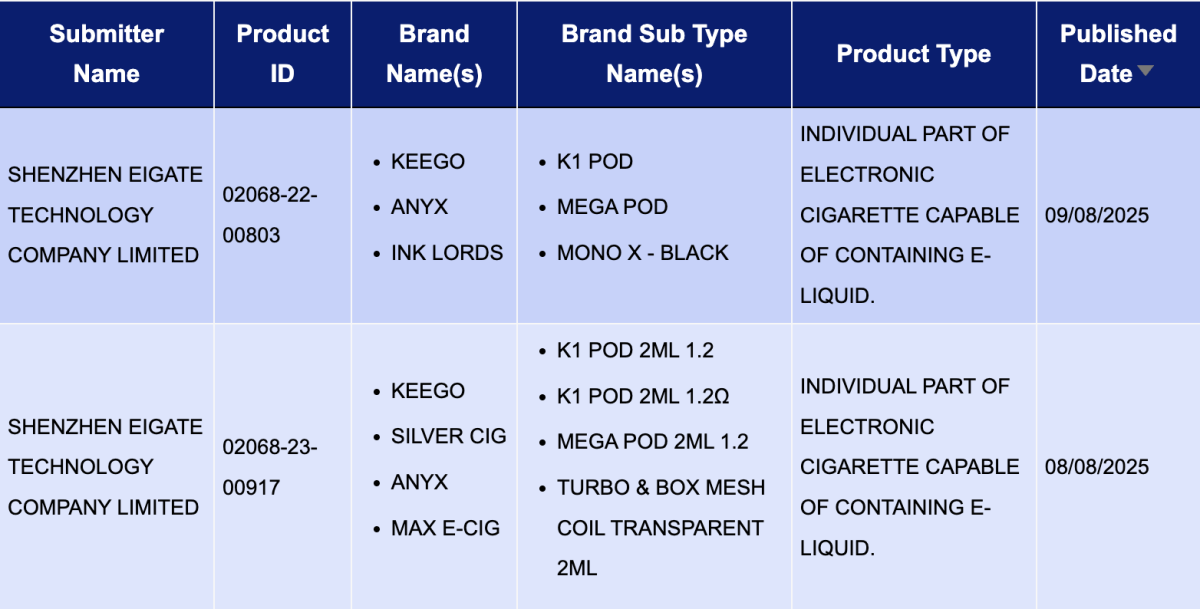
Under the brands of Shenzhen Eigate Technology Co.,Ltd.the following products were published:
K1 Pod, K1 Pod 2ml 1.2 (KEEGO)
MEGA Pod (ANYX)
MEGA Pod 2ml 1.2 (ANYX)
MONO X – Black (INK LORDS)
K1 Pod 2ml 1.2Ω (SILVER CIG)
Turbo & Box Mesh Coil Transparent 2ml (MAX E-CIG)
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







