2022 Premarket Tobacco Product Marketing Granted Orders
2022 Premarket Tobacco Product Marketing Granted Orders
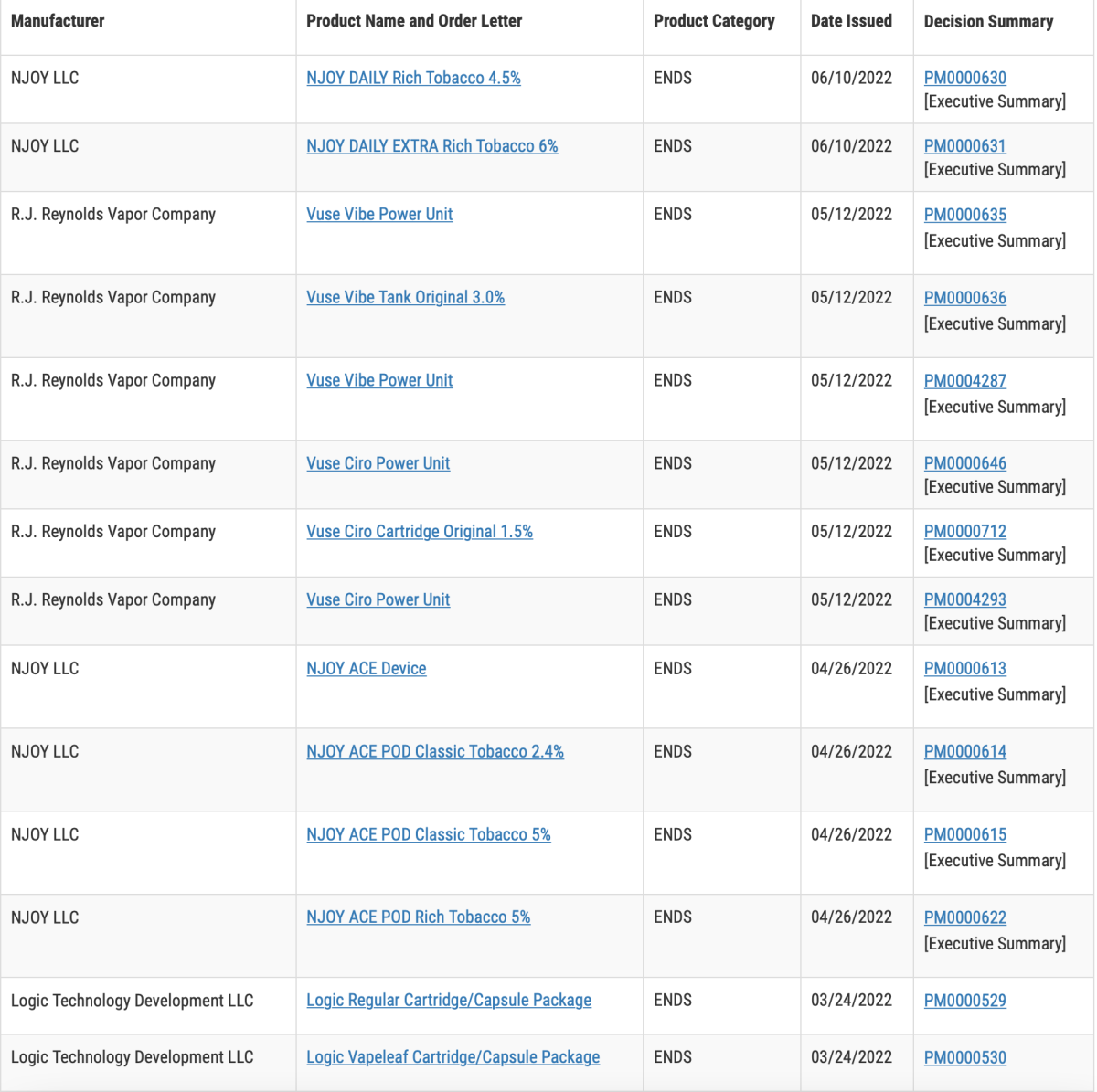
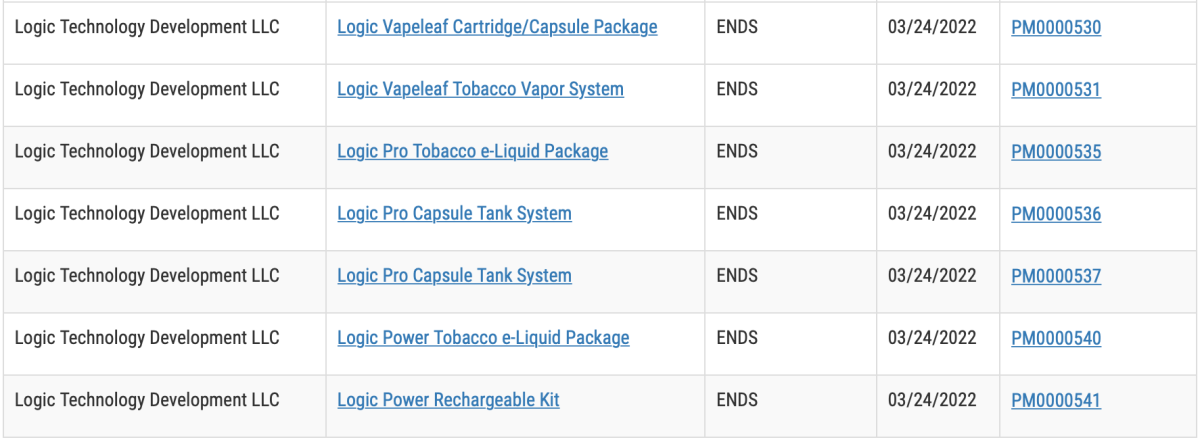
Before making documents available to the public, FDA must redact trade secret and confidential commercial information (CCI) and ensure documents posted to the FDA website are accessible to everyone. For these reasons, the full decision summary for marketing authorizations may not be posted to the website until after the order issuance date. In the interim, to provide as much information as possible at the time of order issuance, we are making redacted versions of the order letter and the “Executive Summary” section of the decision summary available to broadly explain the public health rationale for authorization of these products.
Source:FDA







