
Special statement:
This article is for internal industry communication only, and does not make any recommendations for brands or products.
The images presented in this article are solely used to depict facts and are not intended as advertisements for any products.
Minors are prohibited from accessing this article.
Shenzhen-based tech company Sikary Technology Co., Ltd. has released its first refillable oil pod e-cigarette under the brand MEMERS VAPE on its official website - the MEMERS Dr. AIR series. The series is equipped with the Dr. AIR app, with the brand claiming it to be the "world's first AI-driven e-cigarette solution.
According to two sources, since December 2024, multiple e-cigarette brands including VOZOL and GEEKVAPE have launched high-definition screen e-cigarette products with refillable cartridge capabilities. The specifications are listed in the table below.
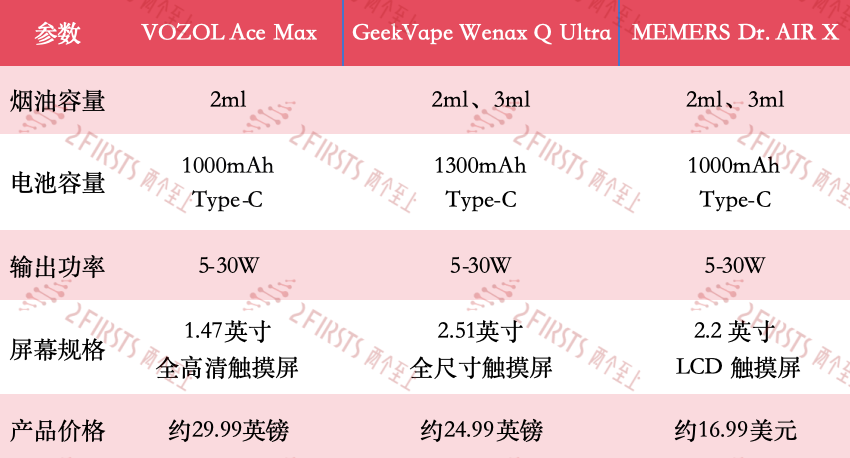
By comparing the new pod-style refillable e-cigarette products released by VOZOL, GEEKVAPE, and MEMERS VAPE, it can be observed that all of these products are equipped with larger sized display screens.

Among them, the VOZOL Ace Max is equipped with a 1.47-inch touchscreen that displays data such as power, number of puffs, and battery life. The GeekVape Wenax Q Ultra, on the other hand, has a larger 2.51-inch screen and supports custom UI themes, product functions, output power, and wrist wake-up functions. The MEMERS Dr. AIR X, which features a 2.2-inch LCD touchscreen, comes with the Dr. AIR application, which the e-cigarette website describes as being able to "track user's e-cigarette data and adjust directly from the phone." According to the e-cigarette website vapinghardware, the Dr. AIR series integrates MEMERS VAPE's MITS AI platform, which can track habits and optimize puffs under AI drive.

In addition, all three products are equipped with large capacity rechargeable batteries and adjustable output power ranging from 5 to 30 watts.
It is worth noting that while MEMERS VAPE claims that the MEMERS Dr. AIR series is the "world's first AI-driven e-cigarette solution," the application of AI technology in e-cigarette products is not new. Last August, e-cigarette brand iJoy introduced the iJoy Bar SD40000, which supports AI voice control.

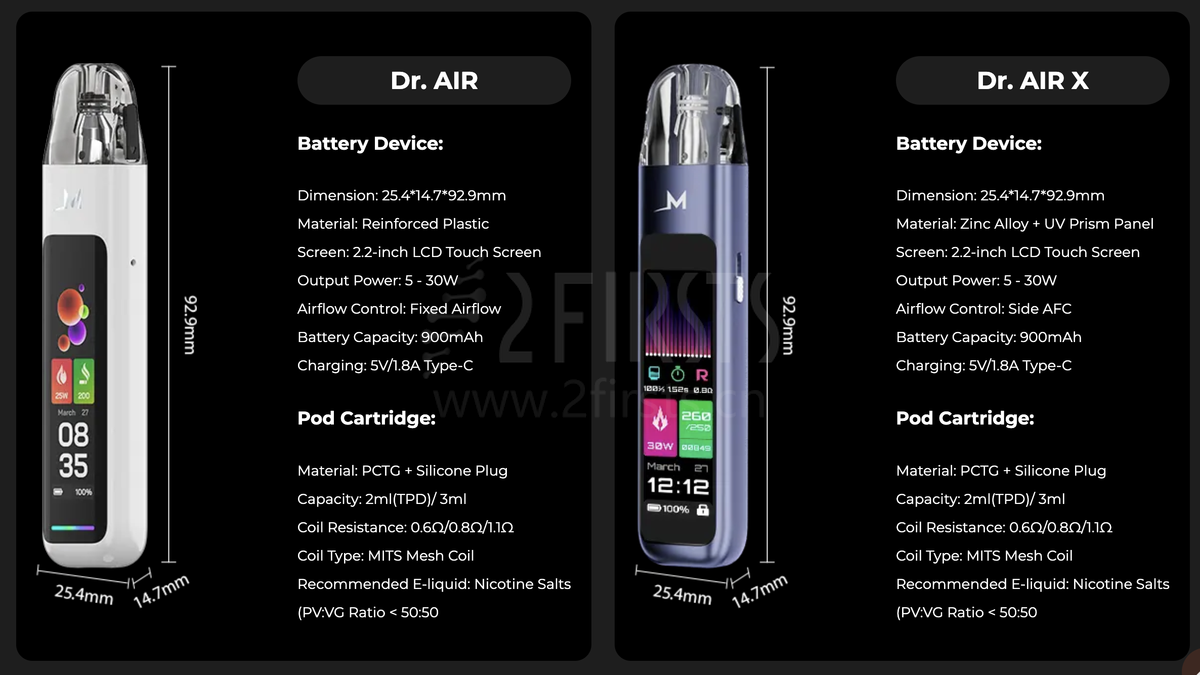
The MEMERS Dr. AIR series of e-cigarettes includes two products, the Dr. AIR and Dr. AIR X. The Dr. AIR X is made of zinc alloy and UV prism material, and has adjustable airflow. Both products can be used with a 2ml (TPD) or 3ml pod, and the official claim is that the pod can be refilled approximately 10 times.
Currently, the MEMERS Dr. AIR series e-cigarette has been listed on the MEMERS VAPE official website and is being sold on e-cigarette distributor websites such as vapesourcing and morevaping, with a retail price of approximately $16.99.
2Firsts will continue to monitor the performance and market trends of e-cigarette products globally, providing the public with accurate and authoritative industry information.
The product section of 2FIRSTS focuses on providing readers with the latest information on new products in the tobacco industry. We welcome readers to contribute and share the latest products in the e-cigarette field to help collect and share first-hand information in the industry.
If you have any unique insights or information, feel free to contact us at any time through the email address info@2firsts.com.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







