According to a report on March 28th, Qixinbao revealed that Shenzhen Wulian Technology Co., Ltd. has been granted a utility model patent for a product called "Simulated Electronic Cigarette".
Basic information on a patent for a simulated electronic cigarette | Image source: Qixinbao
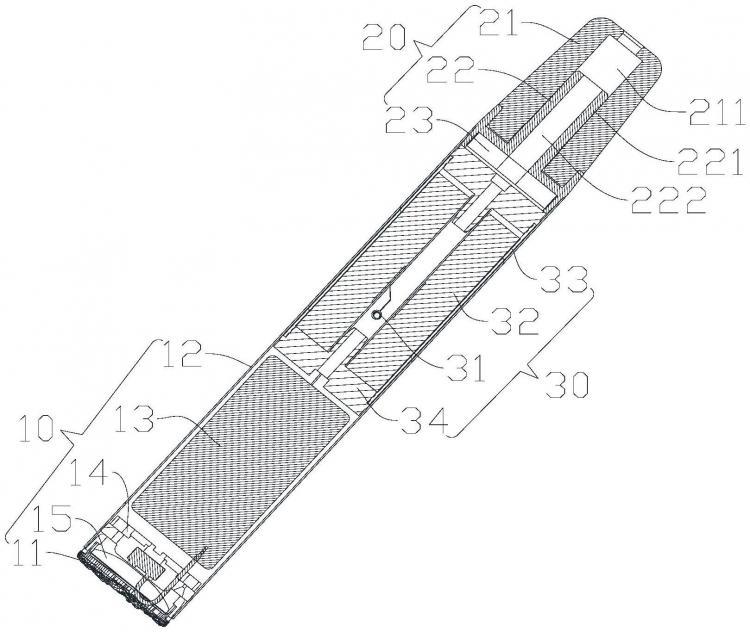
The patent abstract describes a simulated electronic cigarette that consists of a mouthpiece component, atomizer component, and body component. The body component contains an open atomizer chamber, and the atomizer component is located within this chamber. The mouthpiece component is attached to the open end of the atomizer chamber. The body component includes a battery, a light-emitting element, an inhalation detector, and a cigar-shaped shell. The shell contains a light-emitting chamber, a battery compartment, and the atomizer chamber. The light-emitting element is situated within the light-emitting chamber and emits light towards the ridges and grooves on the bottom of the shell. The battery is located within the battery compartment, and the atomizer component is situated within the atomizer chamber. The inhalation detector is located on the side of the battery opposite the light-emitting element and is used to measure the air flow passing through the atomizer chamber. This detector controls the working current of the atomizer component and the luminous intensity of the light-emitting element. The working current of the atomizer component is proportional to the luminous intensity of the light-emitting element.
Patent Abstract with Figure | Image Source: Qixinbao
Reference:
Qi Xin Bao reports that Shenzhen Wulun Technology Co., Ltd. has filed for intellectual property rights.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.







