
Product Highlights:
Battery Life & Usage: The SKE CL2000 comes with a dual-pod setup of 1.8mL + 2.2mL, offering a total of 2,000 puffs—equivalent to about 500 puffs per mL of e-liquid.
Refill Technology: The brand claims it features an “Insert-and-click pod system with auto-refill technology”, combining plug-and-play convenience with automatic refilling.
Flavor Options: Available in 16 classic flavors plus 4 new flavors, including Spring Berry, Choc Mint, Medium Citrus Tart, and Lemon Sour Candy.
Battery Capacity: Equipped with an 850mAh battery—upgraded from the SKE BAR’s 500mAh—for longer-lasting performance.
Market Availability: Listed with the UK Medicines and Healthcare products Regulatory Agency (MHRA) and now available on UK vape distributor websites, retailing at £10.99.
In April 2025, e-cigarette brand SKE launched its small-capacity replaceable-pod product, the SKE BAR, featuring an integrated replaceable pod design. Recently, 2Firsts observed that SKE has introduced a new model—the SKE CL2000—with a total e-liquid capacity of 4mL (1.8mL + 2.2mL). The company claims it delivers up to 2,000 puffs in total.
Claimed to Feature Auto-Refill Technology & Plug-and-Play Pod System, Offering 2,000 Puffs
2Firsts has compiled the product specifications for the SKE CL2000, as shown in the following chart:
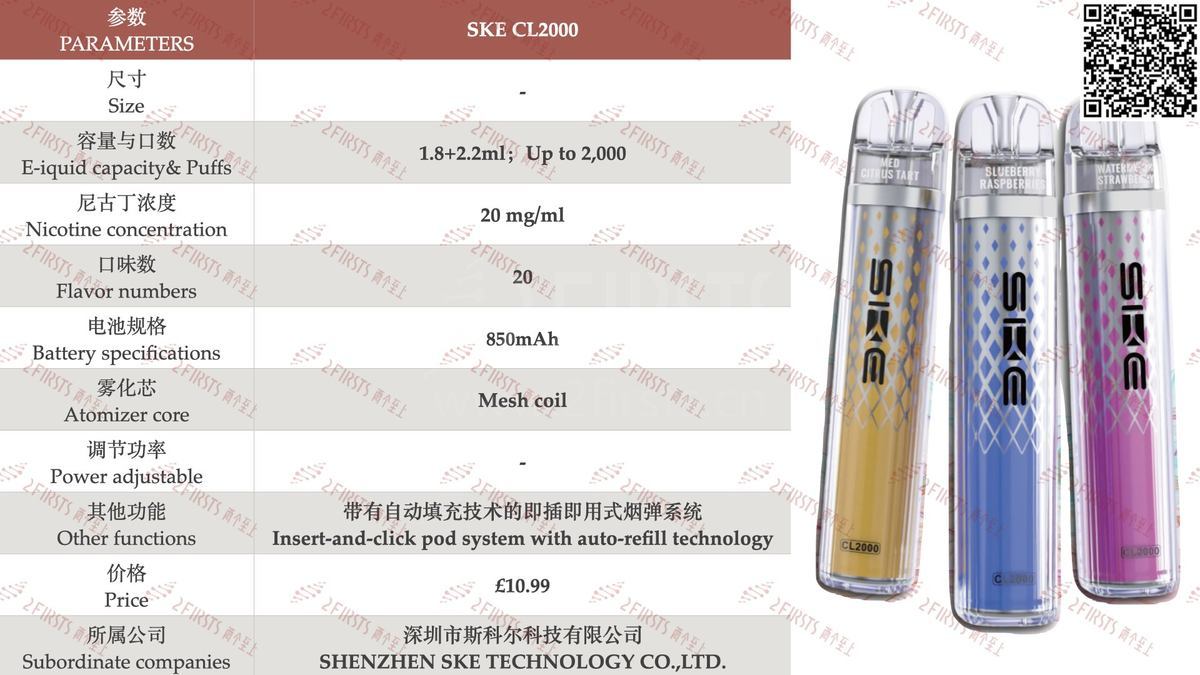
From the specifications, the SKE CL2000 has a total e-liquid capacity of 4mL, consisting of a dual pod setup—one 1.8mL pod paired with a 2.2mL prefilled refill pod. The two pods are connected via a clamp structure. According to the brand, the device features an “Insert-and-click pod system with auto-refill technology”, and is claimed to deliver a total of 2,000 puffs, which equates to 500 puffs per mL of e-liquid.

Endurance experience: The SKE CL2000 is equipped with a 1.8+2.2 milliliter dual pod, with a total suction of up to 2000 puffs, and can deliver 500 puffs per milliliter of e-liquid.
In terms of flavors, the SKE CL2000 offers 16 classic options and 4 new flavors. The new flavors are: Spring Berry, Choc Mint, Med Citrus Tart, and Lemon Sour Fudge.

The SKE CL2000 has a higher battery capacity than the previously released SKE BAR, while maintaining the same nicotine strength of 20mg. Compared with the SKE BAR, which has a 500mAh battery, the new SKE CL2000 is equipped with an 850mAh battery. In terms of flavor range, the SKE BAR offers 35 flavors, whereas the CL2000 provides 20.

In terms of the atomizer coil and nicotine strength, the SKE CL2000 and SKE BAR are the same, both featuring a mesh coil and a nicotine concentration of 20mg.
Listed with MHRA and Now Available via UK Distributors
According to 2Firsts’ search of the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product notification database, the SKE CL2000 DEVICE was officially listed on June 30, 2025.
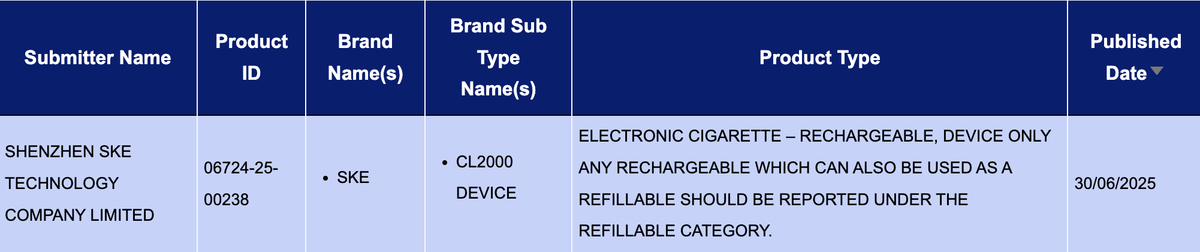
All MHRA-listed product information for e-cigarette brand SKE is submitted by SHENZHEN SKE TECHNOLOGY COMPANY LIMITED, a company founded in 2013 and based in Shenzhen, China. The company also lists other e-cigarette brands under its name, including vfly and MEMERS.
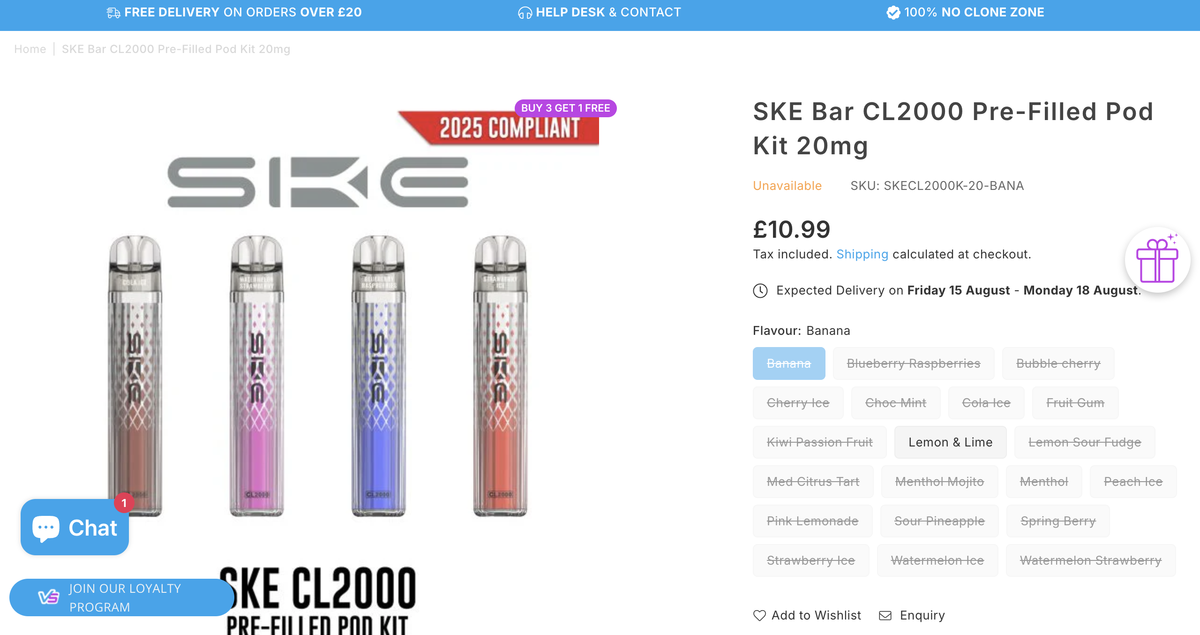
The SKE CL2000 is now featured on the brand’s official website and promoted via SKE’s official Instagram and X (formerly Twitter) accounts. The product has been listed on UK-focused e-cigarette distributor websites such as vape.co.uk and vapestoredirect, marked as “COMING SOON”, with a displayed retail price of £10.99.
As a global leader in NGP media and research, 2Firsts is committed to providing the latest product and technology information and insights across all categories of new generation products (NGPs), including e-cigarettes, heated tobacco, and modern oral products. Our mission is to drive technological transformation and innovation in NGPs worldwide, bringing tobacco consumers safer, reduced-risk products and lifestyles.
With information sources spanning China’s supply chain and global markets, 2Firsts’ product coverage has become one of the most influential platforms for new product and technology releases in the world.
We welcome you to connect with 2Firsts to:
Provide leads on new products or technologies.
Offer reviews or comments on products and technologies.
Seek media coverage for your products.
Gather sales channel information for products.
Contact Information:
Email: info@2firsts.com
Connect with 2Firsts CEO Alan Zhao on LinkedIn
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







