
According to a report by WCBM on September 20th, former U.S. President Donald Trump stated that if re-elected, he would "save" e-cigarette products.
On Friday, the 20th day of the month, Trump posted on his social platform, "Truth Social," stating that...
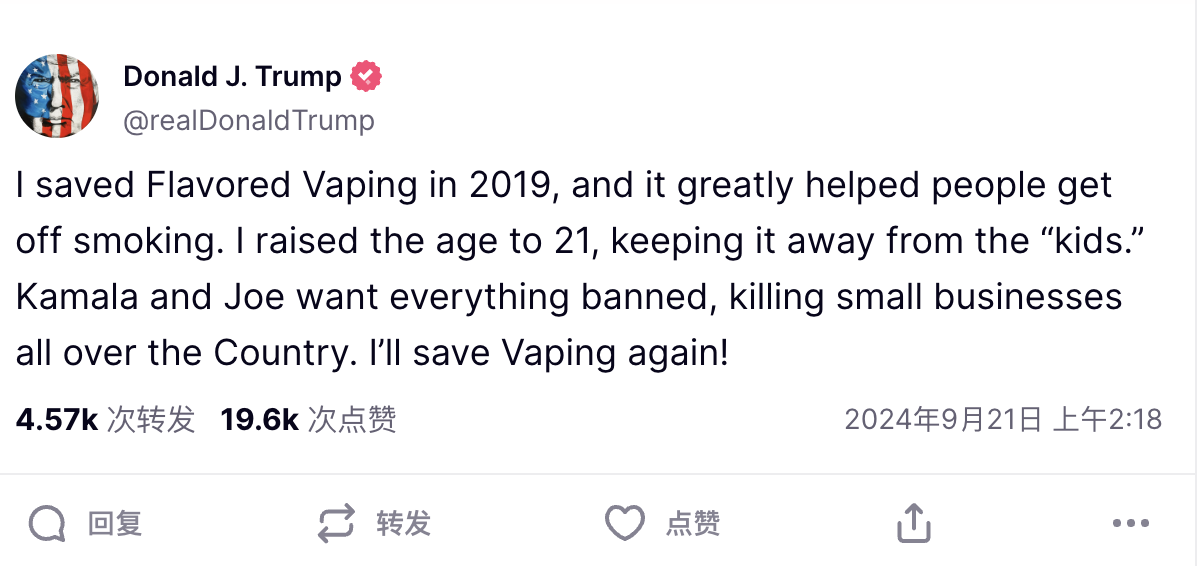
I saved Flavored Vaping in 2019, and it greatly helped people getoff smoking.l raised the age to 21, keeping it away from the "kids.Kamala and Joe want everything banned, killing small businessesall over the Country.I'll save Vaping again!
During his first term, the Trump administration implemented a limited ban on flavored e-cigarettes. Previously, Trump had promised to ban all gas stations and convenience stores from selling flavored e-cigarette products, but two days later, he reversed his stance on Twitter, stating that e-cigarettes can help people quit smoking.
In November 2019, President Trump met with public health organizations, tobacco industry CEOs, and e-cigarette supporters. According to Politico, Trump's proposal includes a 30-day ban on the sale of all sweet and fruity flavored e-cigarette pods and cartridges, while allowing gas stations and convenience stores to continue selling menthol-flavored cartridges and e-cigarettes. Additionally, the proposal allows e-cigarette shops to continue selling e-cigarette e-liquids for open system devices, which are primarily used by adults.
According to Politico, supporters of the free market, small e-cigarette shop owners, and e-cigarette manufacturers are endorsing Trump's proposal, while anti-tobacco advocates are accusing Trump of caving in.
The president of the anti-tobacco organization, Matthew Myers, stated in a press release that this is a surrender to Juul and e-cigarette stores and a green light for the e-cigarette industry to continue targeting children with flavored products and fueling their addiction.
As California Attorney General, current Vice President Kamala Harris previously supported a bill aimed at banning minors from using e-cigarettes.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







