
Special Disclaimer:
- This article is intended solely to explore the policy trajectory of U.S. e-cigarette regulatory authorities. The collection and analysis of information regarding relevant political figures are confined strictly to statements made within the e-cigarette regulatory domain.
- This document does not address any political information, views, or commentary outside the scope of e-cigarette regulation.
- Media sources cited herein represent only the views of the respective source publications. Citations within this document are limited to research on e-cigarette regulation and do not imply any commentary on the political orientation or stance of any cited media source.
On November 6, 2024, U.S. time, Donald Trump won the presidential election, securing his return to the White House. Trump's victory was largely attributed to his strong campaign team, which included not only celebrity entrepreneurs like Elon Musk but also a political star: Robert F. Kennedy Jr., a member of the Kennedy family.

U.S. media report that Donald Trump’s presidential victory could position Robert F. Kennedy Jr. for a key role in health care under the new administration. Previously, CNN disclosed that Kennedy had informed his supporters of Trump’s promise to appoint him to oversee several public health agencies, including the Department of Health and Human Services (HHS) and the Department of Agriculture (USDA). If fulfilled, this would extend Kennedy’s influence to the Food and Drug Administration (FDA), a major agency regulating e-cigarettes in the U.S.
Kennedy, a scion of the influential Kennedy family—with his father, Robert Kennedy, a former congressman, and his uncle, John F. Kennedy, one of America’s most iconic presidents—appears set to make waves in the health sector. According to The New York Times, Trump has encouraged Kennedy to pursue impactful changes in public health. Kennedy also stated this week that "entire departments" within FDA "have to go."
With Trump and Kennedy seemingly intent on reshaping the U.S. health care system, questions arise over whether these reforms could extend to e-cigarette regulation, potentially affecting the PMTA (Premarket Tobacco Product Application) process. 2Firsts has reviewed his past statements to assess their potential stance and approach toward tobacco regulation.
Kennedy: FDA Is More Focused on the Interests of Large Pharmaceutical and Food Companies Than on Public Health
The New York Times described Kennedy in a report as a "Foe of Drug Makers and Regulator," noting his previous statements about wanting to overturn the nation's agricultural system and public health bureaucracy, and overhaul the entire regulatory framework.
On October 9, Kennedy posted a video on his Facebook account criticizing FDA, accusing it of failing to fulfill its responsibilities. He pointed out that FDA tends to prioritize the interests of large pharmaceutical companies and food corporations over the public's well-being. Despite high public health spending in the US, its results have been underwhelming compared to other developed nations. As a result, he is committed to rooting out corruption within FDA to make the country healthier. Kennedy also established a political action committee under the slogan "Make America Healthy Again" (MAHA).
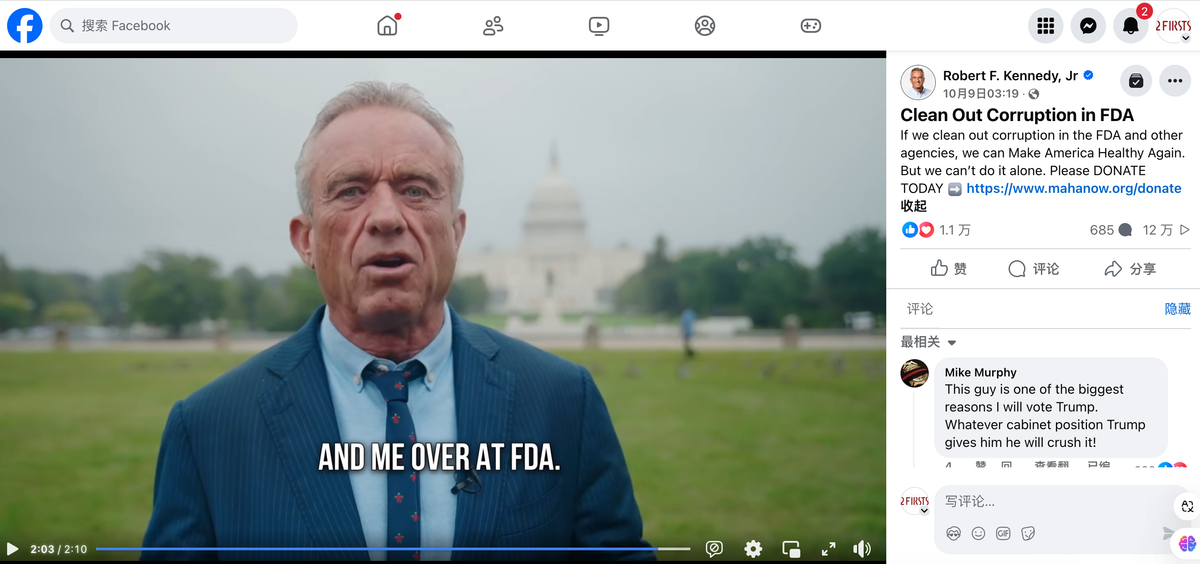
On November 7, Kennedy also expressed his dissatisfaction with FDA during an interview with MSNBC (Microsoft National Broadcasting Corporation). He said, "In some categories of workers, entire departments—like the nutrition departments at FDA—have to go. They are not doing their job; they're not protecting our kids."

Moreover, Kennedy has repeatedly expressed his concerns about the state of public health in the United States. Media reports highlight that Kennedy’s worldview is encapsulated in two of his statements: "For most of the healthcare system, nothing is more profitable than a sick child" and "Public health agencies have become puppets of the industries they were meant to regulate."
Kennedy Raises Concerns over Youth Appeal of NGPs
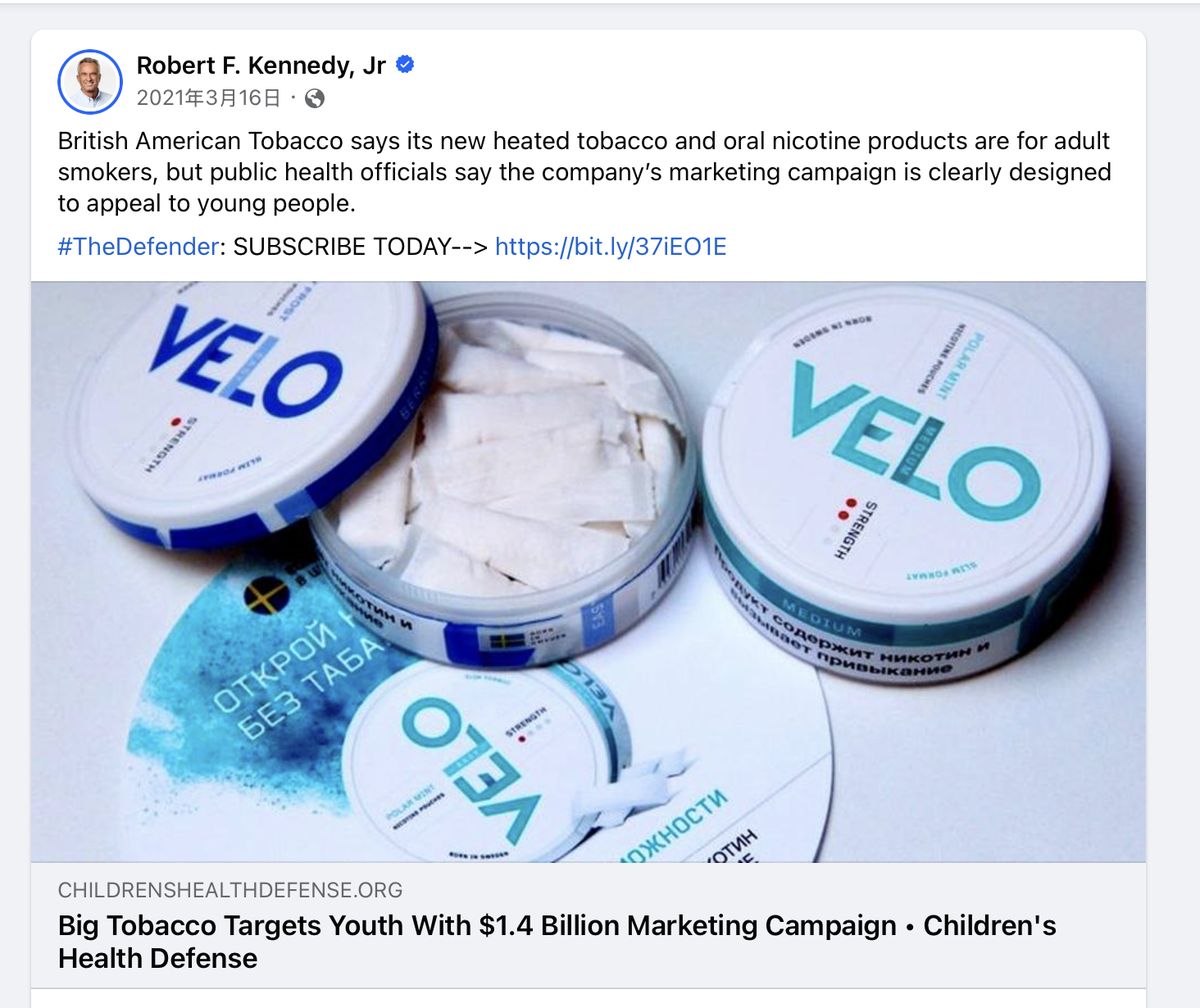
Robert F. Kennedy Jr. has turned his attention to the appeal of new generation products (NGPs) among minors. In March 2021, he highlighted British American Tobacco’s (BAT) nicotine pouch brand VELO in a Facebook post, noting:
“British American Tobacco claims its new heated tobacco and nicotine pouch products are intended for adult smokers, but public health officials argue that the company's marketing clearly targets young audiences.”
This statement underscores Kennedy's scrutiny of industry practices and his focus on preventing youth exposure to nicotine products.
Outlook: With Kennedy’s Expected Role in Health Care, Major Reforms in U.S. E-Cigarette Regulation May Be on the Horizon
Immediately following Trump’s election win, 2Firsts spoke with Tom Beaudet, CEO of U.S. compliance firm Accorto, to assess potential shifts in e-cigarette regulation under the new administration. Beaudet noted that despite receiving millions of applications, the current administration has approved only nine new reduced-harm products over the past four years. He predicts that FDA under the next administration may work to establish a clearer pathway for market access, facilitating faster approvals for companies that submit comprehensive scientific data.
Whether Kennedy will ultimately lead the U.S. health care system and the nature of any reforms he might implement remain to be seen. However, given his past comments on FDA and the tobacco industry, Kennedy is expected to champion significant reforms, with a focus on protecting minors and closely regulating major tobacco companies. With the Republican Party controlling both chambers of Congress and Trump unencumbered by re-election concerns, Kennedy could pursue reform initiatives with unprecedented momentum if he secures Trump’s support.
2Firsts will continue monitoring developments in U.S. e-cigarette regulation and market trends.
For information and opinions regarding the regulatory policies of e-cigarettes in the United States, readers are welcome to share their thoughts by emailing us at info@2firsts.com. Outstanding submissions or viewpoints will be published on the 2Firsts platform.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







