
According to a report from VapingPost on May 14th, the World Health Organization (WHO) recently released a report showing that the number of adolescent e-cigarette users has surpassed smokers. The report expresses concern about this phenomenon.
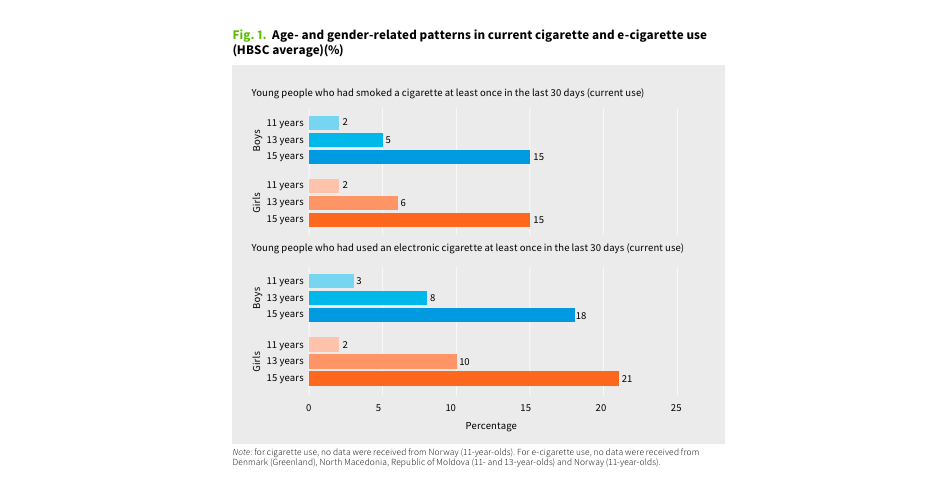
The survey, entitled "Health Behavior in School-aged Children" (HBSC), aims to monitor substance use among 11, 13, and 15-year-old adolescents from 44 countries and regions in Europe, Central Asia, and Canada, including smoking, e-cigarette use, alcohol, and cannabis. This survey is conducted every four years. The latest survey data is from the period of 2021 to 2022, and the results show that e-cigarette use has surpassed traditional tobacco use.
According to the study, among 11-year-old teenagers, the proportion who have smoked at least one cigarette in the past 30 days is 2%, while the proportion who have used an e-cigarette is 2.5%. For 13-year-olds, these proportions are 5.5% and 9%, and for 15-year-olds, they are 15% and 19.5% respectively.
The World Health Organization has expressed concern over this situation, calling for special attention to the issue of e-cigarettes and recommending a "ban or strong regulation" on their use, as e-cigarettes are primarily marketed towards youth. The organization also proposes taking action by "banning advertisements, promotions, and various flavored e-liquids, to reduce the accessibility of e-cigarettes to young people.
The World Health Organization maintains its stance against e-cigarettes and has outlined a precise increase in the number of young people using e-cigarettes. However, their description of regular smokers is more vague, only noting a decrease in the "number of smokers in people's lives." To obtain specific data, one must look at the report's appendix and compare it to the previous survey. The appendix indicates that the percentage of 15-year-olds smoking has decreased from 28% in 2018 to 25% in 2022, a decrease of nearly 11%.
Some observers criticize a recent report that despite numerous scientific studies verifying that e-cigarettes pose less harm to health than traditional tobacco, the World Health Organization has chosen to condemn e-cigarettes. The rise of e-cigarettes may not only lead more young people to nicotine through e-cigarettes instead of traditional tobacco, but also potentially decrease the number of young people who have tried smoking. In other words, young people are increasingly leaning towards choosing relatively healthy options when experimenting with substances, and the WHO should welcome this result rather than criticize it.
The World Health Organization strongly opposes e-cigarettes, believing they are primarily designed to entice young people into addiction to nicotine products. The World Health Organization encourages countries looking to combat smoking to support alternative nicotine delivery methods.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








