Disclaimer: This article is only reprinted for the purpose of industry research and does not represent 2Firsts' endorsement of information and opinions found in other reports from the original media.
Key points:
·Conditional approval: The first batch of heated tobacco products in Taiwan, China has received conditional approval, with companies needing to meet various conditions, including funding third-party monitoring, reporting on usage data, and maintaining a system for reporting adverse events.
·Regulatory upgrades: The revised Tobacco Hazards Prevention Act completely bans e-cigarettes and introduces a health risk assessment mechanism for new tobacco products, strengthening supervision of heated tobacco products.
·Market impact: The entry of heated tobacco products into the market will depend on further reviews and product packaging, with concerns being raised about their market prospects and impact on the traditional tobacco market.
·Protection of vulnerable groups: The review committee attaches special importance to protecting the rights of minors, pregnant women, and other vulnerable groups, requiring companies to avoid misleading advertising.
·Companies suspected of approval: While the two approved companies have not disclosed their full names in official documents, FTNN news directly identifies them as "Philip Morris International Limited" and "Japan Tobacco International Limited" in their reporting.
On July 29, according to a report by Focus Taiwan, the Health Promotion Administration (HPA) in Taiwan announced that the first batch of heated tobacco products has been conditionally approved for legal sale under the amended Tobacco Hazards Prevention Act.
The amended Tobacco Hazards Control Act will take effect on March 22, 2023, completely banning e-cigarettes and introducing a health risk assessment mechanism for new tobacco products such as heated tobacco. These products must undergo review before they can be produced, imported, or sold.
According to the HPA, a total of two companies have been conditionally approved to produce 14 types of heated tobacco products.
The agency stated that it has issued notices to both companies on that day, indicating that the time for the products to enter the market will depend on further review and product packaging.
At the same time, conditional approval does not equate to a simple pass, and companies submitting products must regularly provide reports or face the risk of approval being revoked. The approval process will follow the same procedures as traditional tobacco products, including a review of packaging and product ingredients.
The Health Promotion Administration will discuss the support measures for personal imports of heated tobacco products and the collection of tobacco surcharges with relevant departments, but these measures will only be implemented after the current administrative review is completed.
These companies must meet seven criteria in order to retain their approval, including funding third-party monitoring, reporting usage data to the Department of Health and Welfare, and maintaining a system for reporting adverse events.
The HPA stresses that companies must also disclose new addictive findings, resubmit changes for review, strictly enforce age verification and advertising controls, and refrain from using misleading statements such as "safer than cigarettes" on packaging.
According to a report by United News, two companies are offering a total of 14 items, including 8 types of pods from company A and 3 accompanying devices items, as well as 6 types of pods from company B and 1 accompanying devices item.
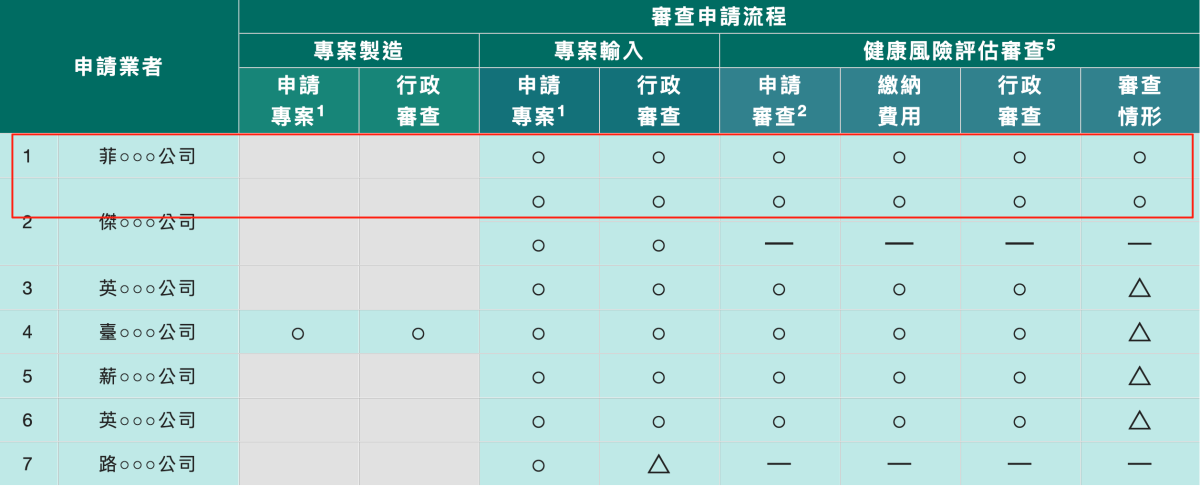
It should be noted that while the two approved companies did not disclose their full names in official documents, FTNN news website directly identified them as "Philip Morris International Inc." and "Japan Tobacco International Inc." in its report.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







