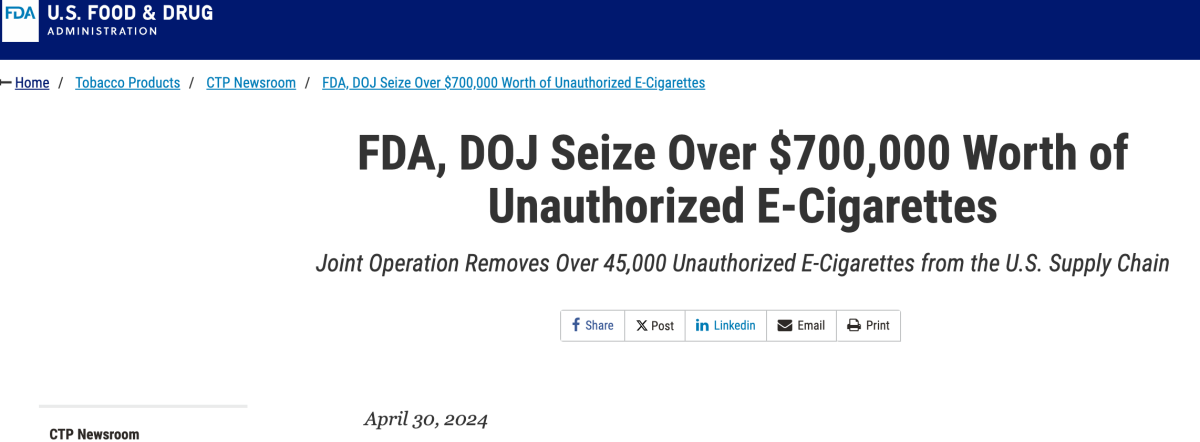
According to an announcement released by the FDA on April 30, the FDA, in collaboration with the DOJ, announced a joint operation with the U.S. Marshals Service to seize illegal e-cigarette products valued at over $700,000. This batch of e-cigarettes was seized at a warehouse in Alhambra, California, believed to be owned by multiple California distributors. The seized products mainly consisted of flavored e-cigarette products, including disposable e-cigarettes from brands such as Puff Bar/Puff, Elf Bar/EB Design, Esco Bar, Kuz, Smok, and Pixi.
This operation marks the first joint effort between the FDA, DOJ, and law enforcement agencies to crack down on illegal tobacco products. A total of over 45,000 illegal e-cigarettes were seized, valued at approximately $703,000.
This seizure initially targeted products held and sold by MDM Group, a distributor operating under the name Eliquidstop.com. In May 2023, the FDA issued a warning letter to MDM, initiating an investigation into their sale or distribution of illegal, flavored e-cigarette products. In January 2024, the FDA conducted a second inspection of the company and found that they were still selling illegal products on a commercial scale. During the raid on MDM facilities, authorities received information that several other companies may be connected to the seized illegal e-cigarettes. On April 5, 2024, the Los Angeles Federal Prosecutor's Office on behalf of the FDA filed a civil forfeiture lawsuit in the United States Central District Court, alleging that the seized products violated federal law.
Jill Atencio, Acting Director of the FDA Compliance Office, stated, "Removing illegal tobacco products from the supply chain is one of our many enforcement tools. The FDA will continue to take appropriate actions against illegal tobacco products, as well as those involved in manufacturing, distributing, importing, or selling these illegal e-cigarette products, especially those that are appealing to young people."
As of April 2024, the FDA has sent approximately 670 warning letters to companies that manufacture and/or distribute illegal e-cigarette products, and has issued over 550 warning letters to retailers selling illegal e-cigarettes. The agency has filed civil penalties complaints against more than 50 e-cigarette manufacturers and over 100 retailers, and has filed permanent injunction complaints against 7 e-cigarette manufacturers.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com