
On October 16, 2024, 2Firsts reported that a product from ZHUHAI QISITECH CO., LTD had been added to FDA's Import Alert 98-06 list, drawing significant attention across the supply chain and among market participants. The announcement raised concerns within the industry, as stakeholders sought clarity on the implications for regulatory compliance and market access.
To better understand the U.S. regulatory landscape for e-cigarettes, 2Firsts reached out to FDA, which provided key disclosures and insights into the agency's enforcement measures.
1. Reason for Listing on Import Alert
FDA listed ZHUHAI QISITECH CO., LTD's RAZ DC25000 due to its status as a new tobacco product that do not have the required FDA marketing authorization.
2. Scope of Impact
A product included on an Import Alert may be detained without physical examination, and future shipments of the product may be detained without having to test or otherwise physically examine it.
3. STN Assignment vs. Authorization Explained
FDA assigns a Submission Tracking Number (STN) to submissions that are received from an applicant, such as a premarket tobacco product application (PMTA). However, an STN does not mean that the product has received authorization from FDA. For a new tobacco product to be legally marketed, it must have FDA authorization.
4. FDA Product Code Classifications
FDA explained to 2Firsts that the product codes are part of its product coding system used during import entry. These codes help classify products by type, such as smokeless tobacco or components/parts of an e-cigarette. FDA decides whether to apply a broad industry code (like "98") or a more specific class code (like 98L or 98M).
However, the classification does not affect the enforcement action. All products listed under Import Alert, including ZHUHAI QISITECH CO., LTD's RAZ DC25000, are subject to detention without physical examination regardless of the specific product code.
FDA also emphasized that it has a dedicated website offering tools and detailed information about its product code system. Detailed information on the different parts of the FDA Product Code can be found at this link: Product Codes and Product Code Builder | FDA. This underscores the importance of proper product code.

5. Three Pathways to Market for New Tobacco Products
To legally market a new tobacco product in the United States, a company must receive a written marketing order from FDA. In addition, companies may receive marketing authorization through one of the three pathways.


6. Only 34 E-Cigarettes Authorized for U.S. Sale
FDA also emphasized that, to date, it has authorized 34 tobacco- and menthol-flavored e-cigarette products and devices. These are the only e-cigarette products that currently may be lawfully sold in the United States.
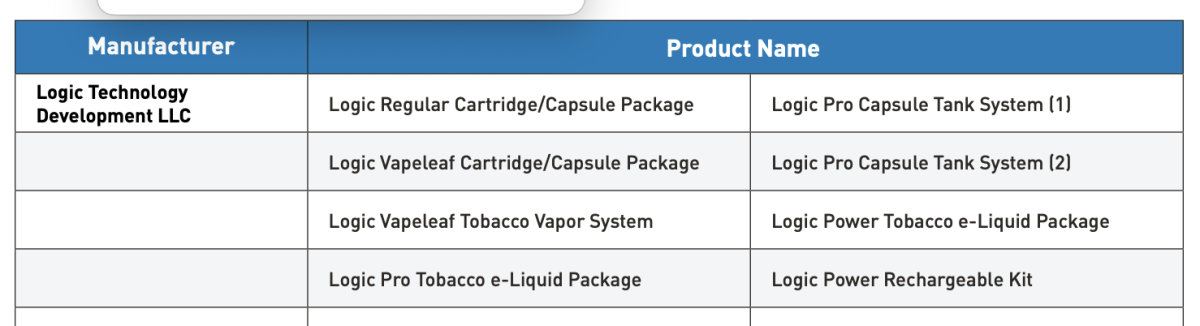
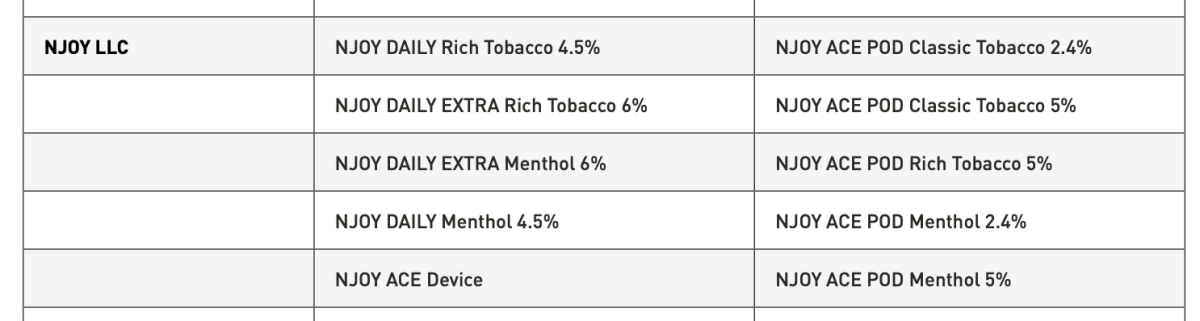
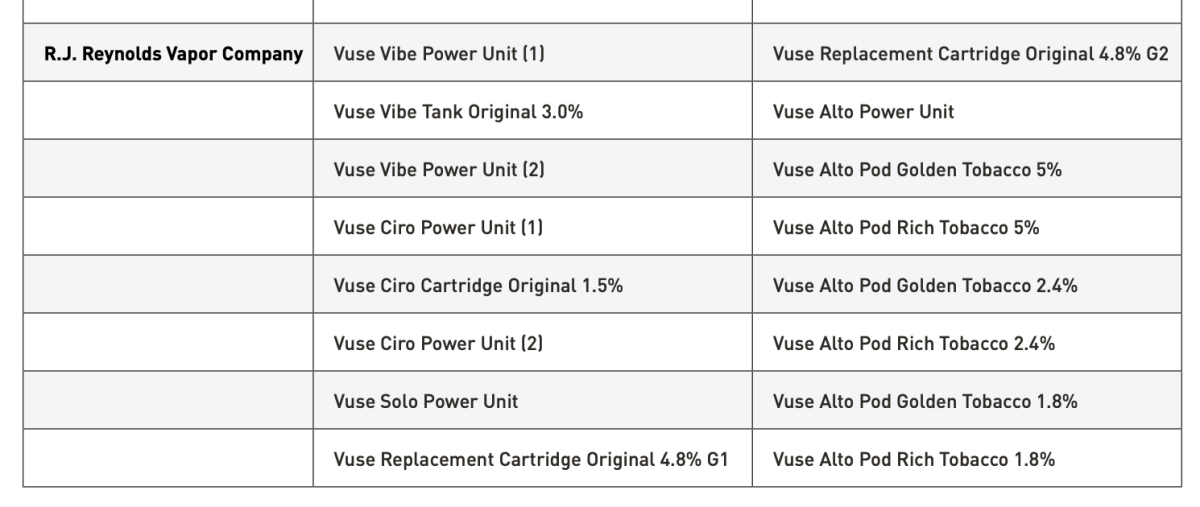
The above information has long been available on FDA's official website and remains available to the public. In its response to 2Firsts, FDA reiterated that these are the only e-cigarette products currently approved for lawful sale in the United States, which further underscores the importance of regulatory compliance for companies seeking to enter the U.S. market.
The e-cigarette industry has clearly felt the increased enforcement efforts of FDA. On October 22, FDA, in collaboration with U.S. Customs and Border Protection, seized 3 million unauthorized e-cigarette products, including GEEKBAR and other brands, with an estimated retail value of $76 million.
2Firsts will continue to follow up and provide the latest reports.







