
The U.S. Food and Drug Administration (FDA) is seeking to impose fines on two brick-and-mortar retailers and 16 online sellers, according to its announcement on November 26. These retailers had previously received warning letters for selling unauthorized tobacco products, but subsequent inspections found they had failed to correct the violations.
The FDA warns that failure to promptly correct violations may lead to additional enforcement actions, including civil fines. The agency emphasized its commitment to addressing unauthorized tobacco products across the entire supply chain. To date, the FDA has filed civil complaints against 79 manufacturers and 175 retailers for distributing or selling unauthorized tobacco products. Furthermore, the FDA is working closely with federal law enforcement agencies, including through the newly formed federal interagency task force. As part of this effort, the FDA and U.S. Customs and Border Protection recently announced the seizure of $76 million worth of illegal e-cigarettes.
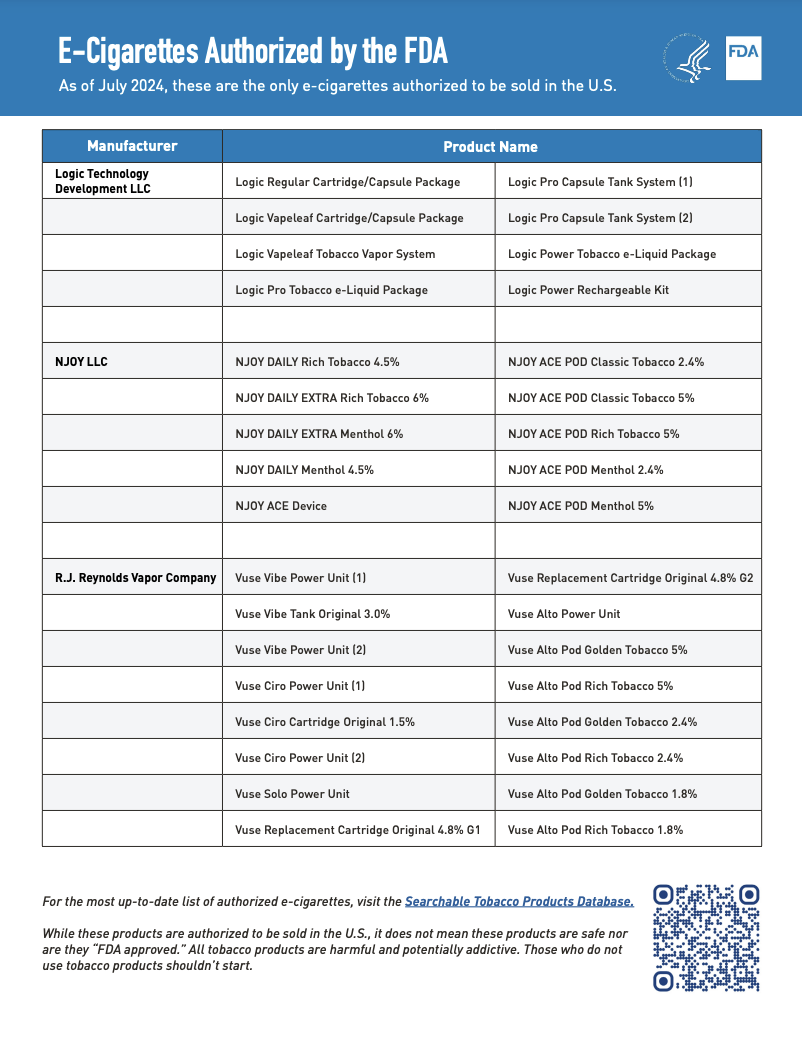
As of the publication date, the FDA has approved 34 e-cigarette products and devices. The agency has also prepared a one-page printable flyer listing all authorized e-cigarette products, which retailers can easily consult to verify which products can be legally sold in the United States. Entities involved in the manufacturing, importing, selling, or distributing of e-cigarettes may face enforcement actions if they fail to obtain the required premarket authorization.








