
October 24, 2025 — 2Firsts News — JUUL’s major investor James Sagan revealed in an interview with NYNext that the company plans to launch its next-generation e-cigarette, Juul2, in the United States. The new device introduces a dual-layer security system combining an app-based age verification process and hardware-level access control.

According to Sagan, users will be required to complete age and identity verification within a companion mobile app — including the use of facial recognition on compatible smartphones — before the device can be activated. Each Juul2 pod contains a built-in chip that connects to the app via Bluetooth and can only be unlocked by authorized accounts linked to verified users.
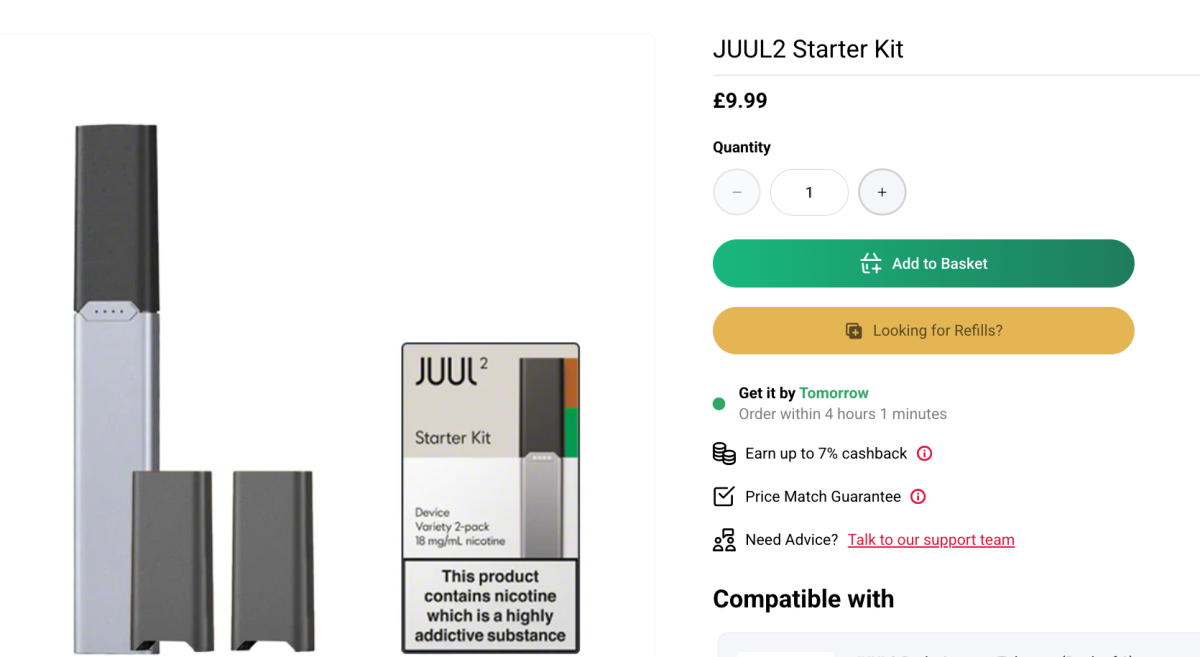

The Juul2 also features a larger-capacity battery, LED display, puff count tracking, and remote firmware update capabilities. The product is currently awaiting regulatory approval in the United States, while the version without biometric safeguards is already available in the United Kingdom.
Sagan noted that JUUL has refocused its business on adult smoking cessation, operating mainly under a B2B model through convenience stores, along with a smaller, age-gated online retail channel.
He added that despite earlier criticism over flavored pods, flavors remain important for helping adult smokers transition away from combustible cigarettes.
“The new product aims to solve three key challenges,” Sagan said. “Keeping e-cigarettes out of the hands of underage users, making the experience more appealing to adult smokers, and ensuring product effectiveness.”
According to Research and Markets, the global smoking cessation market — including e-cigarettes, gums, patches, and other therapies — is valued at nearly USD 35 billion, with major competitors such as Reynolds American (Vuse), Imperial Brands (blu), and Altria (NJOY).
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







