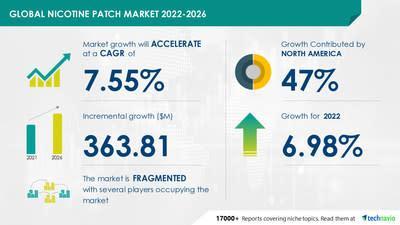
According to a report by Technavio titled "Nicotine Patch Market by Product and Geography - Forecast and Analysis 2022-2026", the nicotine patch market is projected to have a CAGR of 7.55% during the forecast period, indicating accelerated growth. Technavio analysts have classified the global nicotine patch market as a part of the pharmaceutical market within the entire healthcare industry.
Strict regulation is set to be the primary challenge for the nicotine patch market within the forecast period. Participants in the global nicotine patch market must adhere to strict rules and regulations implemented by different governmental bodies in each country to monitor the sale of tobacco products. In the United States, suppliers must comply with regulations set by the FDA. Quit-smoking products are governed by the FDA's Center for Drug Evaluation and Research, which is responsible for ensuring product safety. In India, advertising of nicotine-based products is prohibited.
According to the report, the market share for 24-hour nicotine patches is expected to grow significantly during the forecast period. In 2021, the 24-hour nicotine patch will be the largest product segment in the global nicotine patch market. There are three variants of the 24-hour nicotine patch: 21mg, 14mg, and 7mg. Technological innovations have injected vitality into the global 24-hour nicotine patch market.
The report lists the top five nicotine patch companies, with British American Tobacco Limited being one of them. The company offers nicotine patches under its Velo brand, which contains high-purity nicotine, water, and other high-quality ingredients.
Cardinal Health Inc. delivers nicotine patches including nicotine transdermal systems and 21mg nicotine.
Cipla Health Ltd. offers nicotine patches, such as Nicotex patches, which are transdermal patches that utilize nicotine replacement therapy to aid in smoking cessation.
CVS Health Corp. offers nicotine patches, such as the CVS Smoking Cessation Patch, to aid in smoking cessation.
Reddys Laboratories Ltd. is a company that offers nicotine patches, specifically Habitrol nicotine patches.
In addition, other nicotine patch companies involved in the research include Cigna Corporation, Itaconix Corporation, Merck KGaA, Novartis AG, Perrigo Company Plc, Pfizer Inc., Pierre Fabre SA, Lundbeck Pharmaceuticals Ltd., Target Corporation, Walgreens Boots Alliance Inc., Walmart Inc., GlaxoSmithKline plc, and Johnson & Johnson.
Statement
This article is compiled from third-party information and is intended for industry communication and learning purposes only.
This article does not represent the views of 2FIRSTS and we are unable to confirm the authenticity and accuracy of its content. The compilation of this article is intended solely for industry exchange and research purposes.
Due to limitations in our translation abilities, the translated article may not fully reflect the original text. Please refer to the original text to ensure accuracy.
2FIRSTS maintains complete alignment with the Chinese government regarding any statements or positions concerning domestic issues, as well as those that involve Hong Kong, Macau, Taiwan, or foreign affairs.
The copyright of compiled information belongs to the original media and author. If there is any infringement, please contact us for deletion.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.







