According to 2FIRSTS at the RELX YUEKE e-cigarette physical store in Indonesia, on October 26th Jakarta time, RELX YUEKE officially launched a new product, the RELX Infinity Plus, for sale both online and offline. The new product has a retail price of IDR 300,000, approximately RMB 138, and is claimed to charge up to 80% in just 30 minutes. It comes in five colors: Black Phantom, Lunar Dust, Hidden Pearl, Rising Tide, and Pink Whisper.
The RELX Infinity Plus is now available in five different colors.
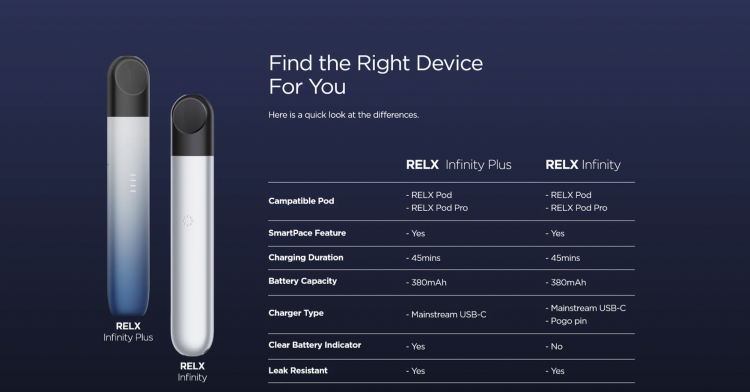
The new product from RELX Yuetheke can now be seen on the official website's homepage. Compared to the old RELX Infinity, it features a clean battery indicator. The standout feature of this new product is its 380mAh battery, which has four levels of remaining power reminders at 25%, 50%, 75% and 100%, providing users with a clearer understanding of their device's battery life. It also has 11 layers of structure to prevent internal leakage and condensation. Additionally, the product can be placed upright, thanks to its flat bottom design, which makes the device easier to stand.
The official website homepage displays that RELX Infinity Plus is releasing new product information, with details about the new product.
Product details, product details, product details.
1. This article is intended solely for internal industry communication and exploration, and does not serve as any form of advertising or recommendation for brands or products. 2. Smoking is harmful to health. Minors are prohibited from reading this article.
This article is an original creation of Shenzhen 2FIRSTS Technology Co., Ltd. The copyright and license to use belong to the company, and any unauthorized copying, reproduction or other infringement of the company's copyright is strictly prohibited. The company reserves the right to pursue legal action against any individual or entity that violates this policy.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.








