By Echo Guo
Co-founder & COO of 2Firsts
Editor's Note:
At the CORESTA 2024 Congress, Dr. Jessica Zdinak, Chief Research Officer at ARAC, presented the company’s latest research. As a leading global media and consultancy in the NGP industry, 2Firsts was intrigued by the findings and obtained the presentation slides to explore their implications. Among the research presented, one study focused on nicotine pouches—already covered in a previous 2Firsts article—and another addressed flavored ENDS, the focus of this piece. Drawing on ARAC’s research and IKE Tech’s recent release on age verification technology, this article examines potential solutions to the societal challenges posed by flavored ENDS.
The United States, the world’s largest e-cigarette market, accounts for approximately one-third of the global share and heavily influences international trends. Over the past year, disposable ENDS with fruit flavors have garnered significant attention for their appeal to minors. This issue has not only dominated media coverage and regulatory discussions but also positioned "flavor" as a central concern under APPH for ENDS.
Currently, only 34 ENDS products have received Marketing Granted Orders (MGO) under the FDA's PMTA, all of which are restricted to tobacco and menthol flavors. These authorized products account for just 13.7% of the U.S. market share, while the majority is dominated by non-compliant flavored products. Bridging this gap requires not only stricter regulatory enforcement but also advancements in research and technology aimed at addressing the complex intersection of flavored ENDS and youth accessibility.
Tackling Youth Access and Smoking Cessation: Insights from LRESS and Age Verification Technology
ARAC presented insightful evidence through a comprehensive study at the 2024 CORESTA. The findings of a longitudinal randomized experimental switching study (LRESS) revealed that flavored disposable ENDS significantly reduced daily cigarette consumption and achieved higher quit smoking rates compared to tobacco-tasting versions.
The research provided important insights into the effects of flavored ENDS on adult smokers but did not address their impact on minors, leaving this aspect outside its scope. Shortly after ARAC released its findings, Ispire Technology Inc. and IKE Tech LLC announced on November 18, 2024, that they had concluded a pre-PMTA submission meeting with the FDA’s CTP. The meeting highlighted their Age Verification technology, designed to restrict youth access to ENDS at the point of use while ensuring adult consumers can access flavored products authorized under the PMTA framework.
Building on these developments, Dr. Zdinak previously shared her insights with 2Firsts during an interview about the FDA's recent memo on PMTA applications for non-tobacco flavored e-cigarettes. She noted, “Age-gating technology may help limit youth access, but its effectiveness in reducing youth use remains uncertain. While various age-verification technologies have emerged, the industry cannot assume that implementing such measures eliminates the need to demonstrate a positive risk-benefit ratio in their PMTA applications.” Her remarks highlight the dual challenge manufacturers face: integrating effective technological safeguards while providing robust evidence that their products meet the APPH standard required for FDA approval.
The continuous advancements in scientific research and technological development are poised to help ENDs return to their original goal of tobacco harm reduction. This progress not only opens up possibilities for resolving the regulatory challenges but also benefits the global NGP industry and the advancement of THR initiatives.
A Rigorous Experimental Design Under the PMTA Framework
The purpose of this study was to provide the FDA with longitudinal experimental data to assess whether candidate disposable ENDS products meet the criteria for being Appropriate for the Protection of Public Health (APPH). Specifically, the study aimed to determine whether these products could successfully facilitate smoking cessation or reduce cigarettes per day (CPD) by more than 50%. Additionally, the study compared the effectiveness of flavored and tobacco-tasting ENDS.
Conducted over six months across four regions of the United States, the study involved participants who were randomly assigned to one of two groups: one group used a combination of flavored and tobacco-tasting disposable ENDS, while the other used only tobacco-tasting disposable ENDS. The experimental design was carefully structured, taking into account participants' smoking behaviors, daily cigarette consumption, and smoking cessation success rates. This approach ensured the scientific rigor and reliability of the findings, providing robust data to support the FDA's review of flavored disposable ENDS products under the PMTA framework.
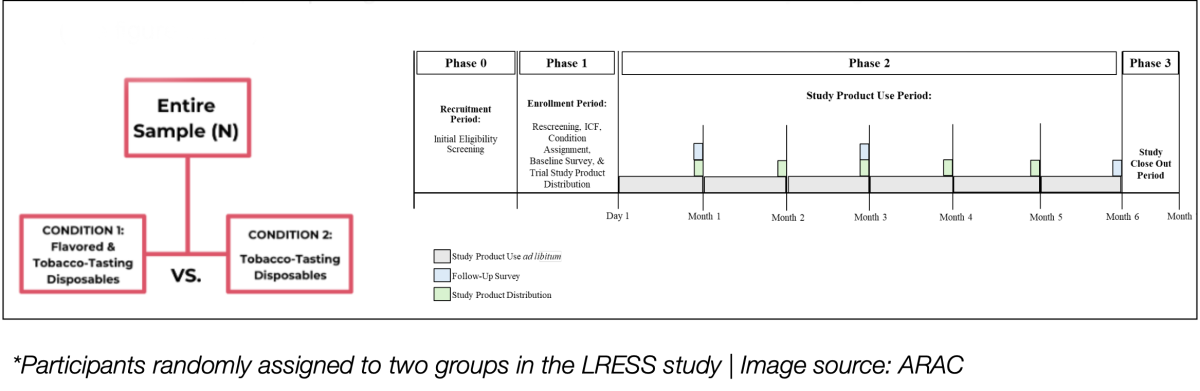
Six-Month Study: Flavored ENDS Outperform Tobacco-Tasting in Boosting Smoking Cessation
ARAC states that this study represents the first known use of a rigorous experimental design that controls for potential confounding variables, recruitment biases, and sample bias. It provides compelling evidence of the increased benefits to the U.S. adult smoker population when a range of both fruit-flavored and tobacco-tasting disposable ENDS products are made available.
The study’s first key finding, integrating the flavored and tobacco-tasting condition with the tobacco-only condition, shows that exposure to disposable ENDS products led to statistically significant reductions in cigarettes per day (CPD) and increased cessation rates over a six-month experimental period.
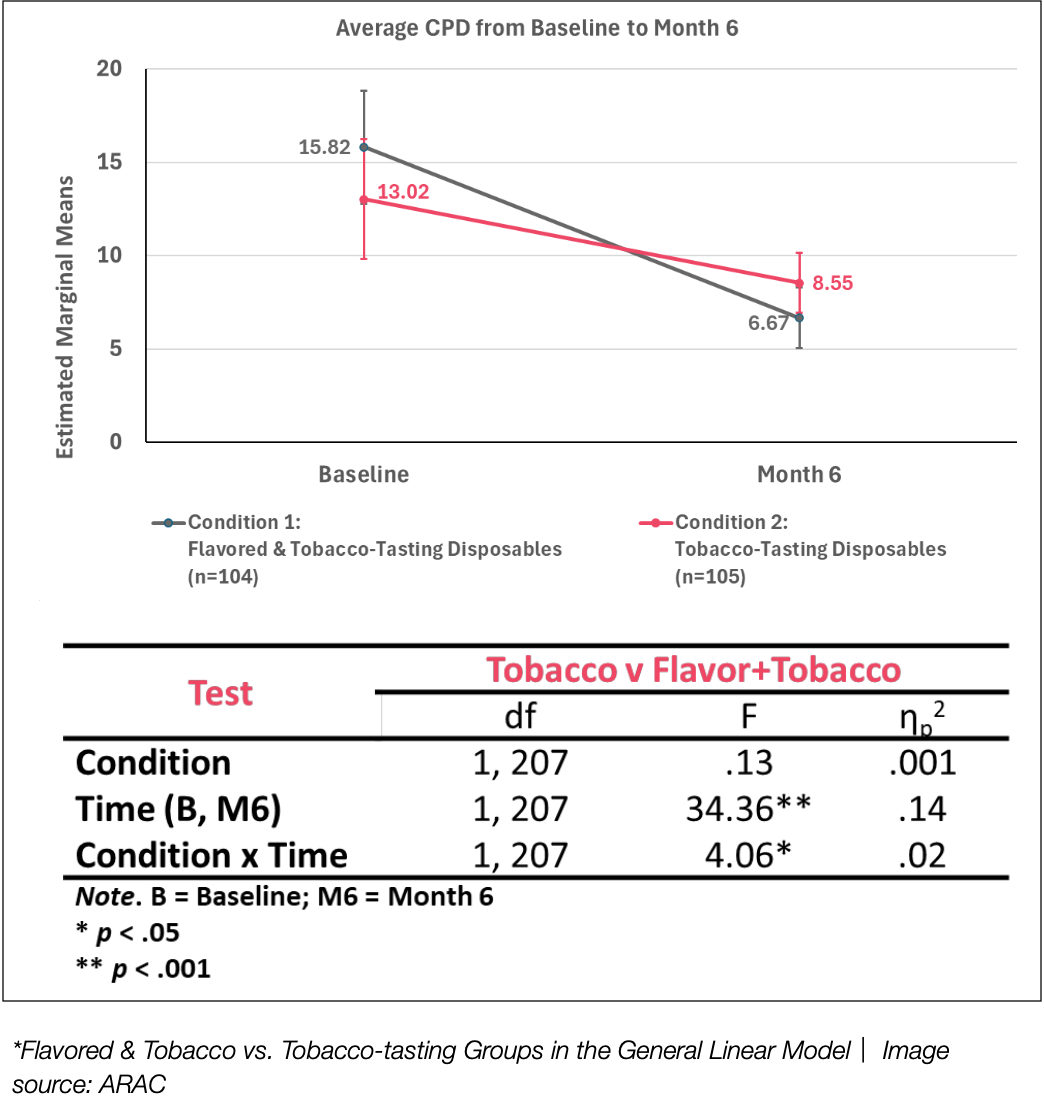
The second conclusion reveals that while both groups significantly reduced their CPD, those using flavored and tobacco-tasting disposable ENDS experienced a greater reduction by month six compared to the tobacco-only group. Specifically, CPD in Condition 1 (flavored & tobacco-tasting) decreased from a baseline of 15.82 to 6.67, switching adult smokers or reducing CPD by > 50%, while in Condition 2 (tobacco-only), it dropped from 13.02 to 8.55.
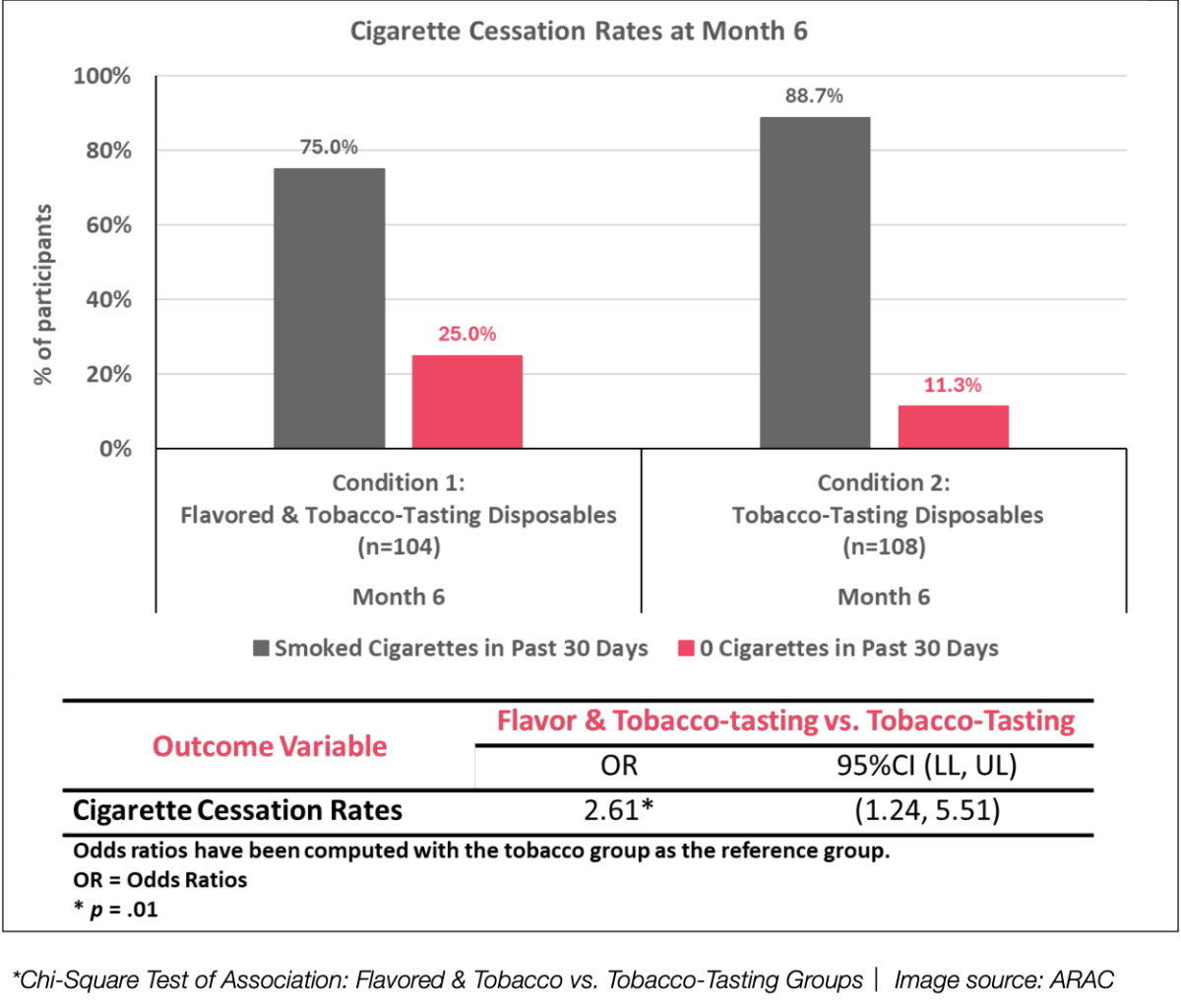
The third key finding reveals that participants using flavored and tobacco-tasting disposable ENDS products were 2.6 times more likely to have quit smoking by the end of the six-month study, compared to those using tobacco-only disposable ENDS. This highlights the enhanced cessation potential of flavored products, further reinforcing the observed greater reductions in cigarettes per day in the flavored group.
Advancing Technology for Social Impact: Age Verification Solutions to Combat Youth Access to Flavored ENDS
While ARAC’s research delves into the APPH-related challenges of flavored ENDS, IKE Tech’s Age Verification system introduces a transformative technological solution. This innovation is particularly critical given that ARAC’s recent LRESS did not examine youth access, leaving a gap that IKE Tech’s technology seeks to address.
In a joint announcement with Ispire, IKE Tech unveiled a Bluetooth-enabled system that connects ENDS devices to a companion app, initiating an automated age verification process. Users are required to submit valid identification, which is encrypted and stored on a blockchain ledger. The decentralized and immutable nature of blockchain enhances data security and authenticity, while integration with government-approved identity verification platforms or authoritative databases confirms users’ eligibility. Only verified adults can activate the device, effectively barring minors from using ENDS.
IKE Tech plans to file a PMTA specifically for its age-gating technology rather than a full PMTA for a finished product. This approach aligns with FDA guidelines, which allow for such submissions if statutory requirements are met. IKE Tech stated in its announcement that the FDA has also signaled its openness to granting priority review for this novel point-of-use technology, potentially expediting its integration into flavored ENDS products. This plug-and-play design could simplify compliance pathways and broaden the approval potential for manufacturers.
To further ease adoption, IKE Tech plans to establish a Tobacco Product Master File (TPMF) for its component. Once authorized, the system and its TPMF will be available for manufacturers to license and incorporate into their devices. This initiative is expected to foster compliance and innovation across the ENDS sector, providing a robust framework for addressing regulatory challenges.
The FDA is tightening enforcement against illicit flavored ENDS, signaling a decisive shift toward stricter regulation. At the same time, behavioral research, such as ARAC’s LRESS, and technological innovations, like IKE Tech’s age verification system, are accelerating the PMTA review process. These advancements pave the way for what could be the first flavored ENDS product to secure a Marketing Granted Order (MGO) under the PMTA framework—a milestone that could reshape the industry.
Such a breakthrough would disrupt a market long dominated by non-compliant products, ending the era where bad actors overshadowed responsible players. As regulatory frameworks take hold, the competitive landscape is likely to undergo significant change, enabling compliant companies to emerge as true market leaders. This transformation promises to usher in a new era of accountability and innovation, setting the stage for a more sustainable and equitable future for the NGP industry.
ARAC BIO
Applied Research and Analysis Company (ARAC) is a leading U.S. based behavioral science research firm that designs, executes, and presents scientifically-sound, yet customizable studies to support manufacturers, regulatory agencies, and industry consultants. Their expertise includes consumer-focused research services in product development and innovation and regulatory science supporting marketing authorization applications, with SUCCESS in U.S. product authorizations and applications.
ARAC specializes in MODULE 5 & 6 studies including: label/claim development and comprehension, human factors/usability testing, and clinical/behavioral studies, such as randomized experimental longitudinal, actual use, TPPI, and post-market surveillance systems.
"Most Outstanding Service to Industry" 2024 Golden Leaf award-winning fully staffed IN-HOUSE psychologists, behavioral scientists, statisticians, survey methodologists, and medical monitoring offer tailored research solutions with unparalleled integrity and an exceptional client experience. (Source: ARAC)
2Firsts is dedicated to reporting on global THR scientific research and fostering deeper and more comprehensive exchanges among science, industry, and regulation. Our mission is to advance the global development of THR initiatives.
We welcome article submissions, interview opportunities, or commentary. Please contact us at info@2firsts.com or connect with 2Firsts CEO Alan Zhao on LinkedIn here.
*This article is an original article of 2FIRSTS Technology Co., Ltd. The copyright and license rights belong to the company. Any entity or individual shall make link and credit 2FIRSTS when taking actions to copy, reprint or distribute the original article. The company retains the right to pursue its legal responsibility.








