By Echo Guo
Co-founder & COO of 2Firsts
Editor's Note:
At the CORESTA 2024 Congress, Dr. Jessica Zdinak, Chief Research Officer at ARAC, presented the company's latest research. As a leading global media and consultancy in the NGP industry, 2Firsts was intrigued by the findings and engaged with ARAC to obtain the presentation slides. This article, authored by 2Firsts, is based on those slides and the key insights from ARAC's research, set against the backdrop of current industry trends. lt explores the study's implications for nicotine pouches, their potential impact on public health, and the regulatory landscape.
In the rapidly evolving market of novel tobacco products, Oral Nicotine Pouches are seen as a promising solution for reducing health risks.
According to the latest insights from Verified Industry, the global market for Oral Nicotine Pouches is projected to surge from $1.2 billion in 2023 to $5.5 billion by 2033. Major players in the tobacco industry, including Philip Morris International (PMI) and Altria, have already secured a dominant position in this rapidly expanding market. Meanwhile, e-cigarette companies, particularly those with supply chains in China, are swiftly entering the nicotine pouch space, aiming to capitalize on this growth and increase competition in the sector. As the market heats up, these developments highlight the growing intersection of traditional tobacco products and newer, potentially less harmful alternatives.
The United States is currently the largest market for nicotine pouches and serves as a barometer for the global market. While the public is increasingly focused on the potential of nicotine pouches to reduce health risks and their convenience of use, there are growing concerns about their ability to appeal to non-users, particularly teenagers. This category also falls under FDA regulation, which requires companies to conduct TPPI(Tobacco Product Perception and Intention)studies as part of the new tobacco product application process, in accordance with the FDA's CTP.
At the CORESTA 2024 Congress, ARAC (Applied Research and Analysis Company) presented a pivotal TPPI study on the public perception of Oral Nicotine White Pouches (ONWP). The study indicates that, compared to smokers, non-users showed lower intentions to try or use ONWP.
Three Key Takeaways: Making the Case for Oral Nicotine White Pouches
One of the key conclusions of the study is the comparison of intentions between users and non-users regarding ONWPs. Non-users showed significantly lower intentions to try both flavored and tobacco-flavored ONWPs compared to users. A crucial aspect of the study also addressed whether flavor or tobacco taste was more appealing to non-users. The findings revealed that non-users intentions to try or use ONWPs were similar for both tobacco-flavored and flavored products.
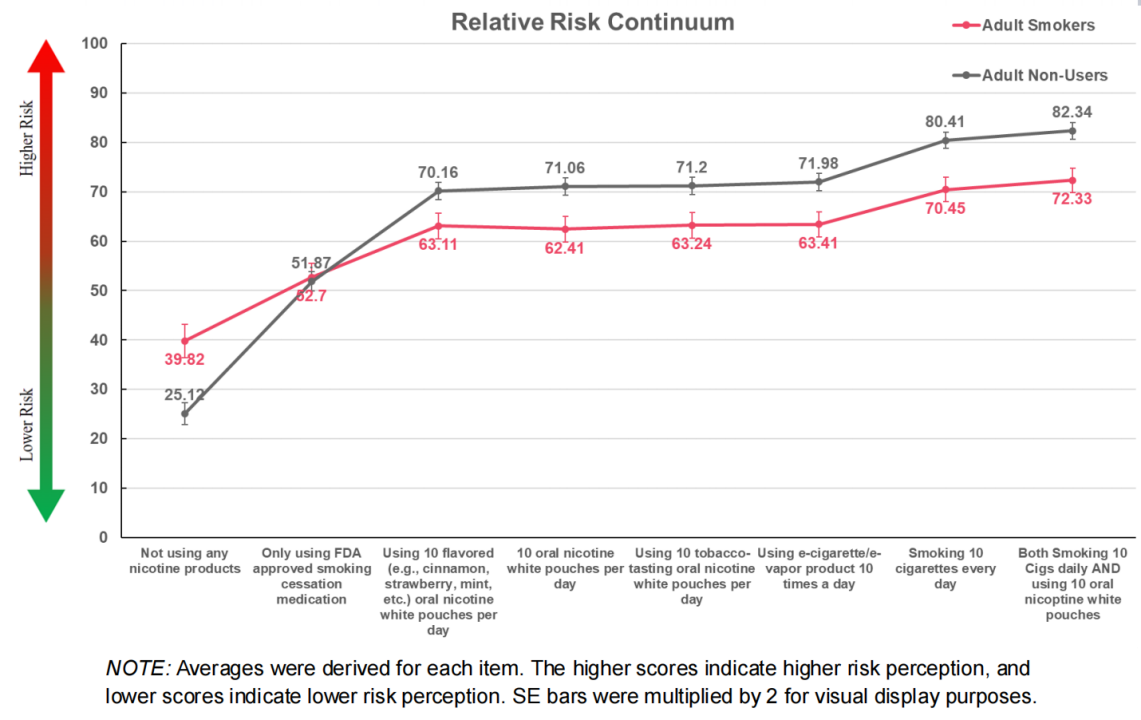
Next, regarding relative risk perceptions, both users and non-users perceived the general pouch category, flavored ONWPs, and tobacco-flavored ONWPs as carrying similar levels of health risk. For most participants, the perceived health risk of ONWPs was lower than that of cigarettes, while others considered the risk of using ONWPs to be on par with smoking cigarettes. This suggests that, in terms of risk perception, ONWPs are viewed more favorably.
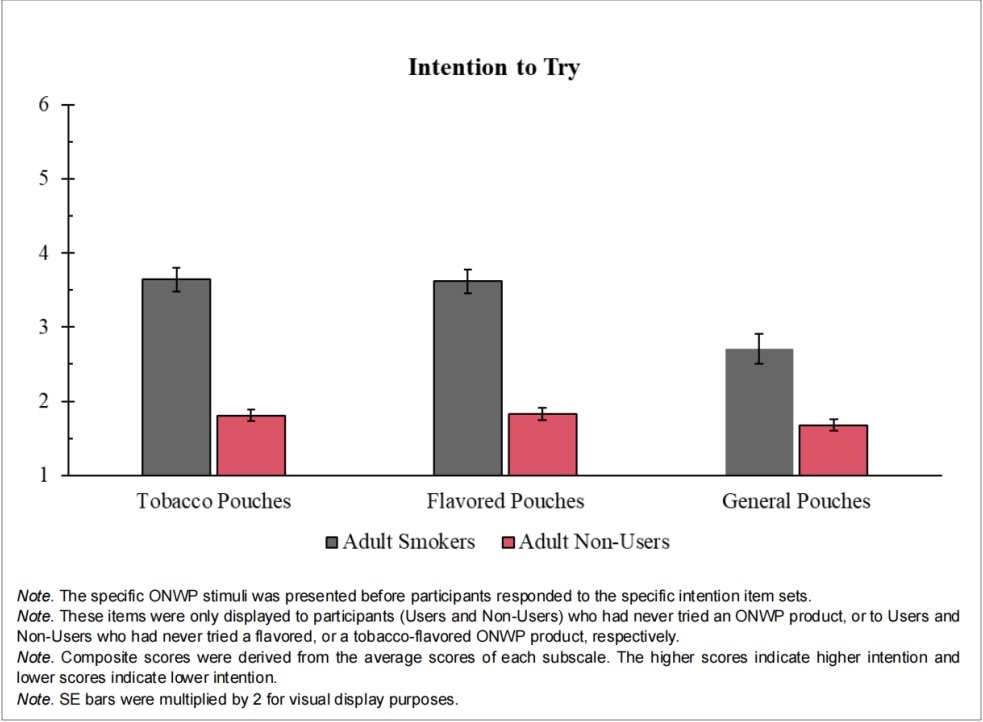
Third, Reasons to Use ONWPs highlighted that the primary appeal is the perception of lower harm compared to smoking. Both the user and non-user groups endorsed the belief that "using ONWPs is less harmful to me than smoking" as the strongest reason for use. While the top reasons for using ONWPs varied between groups, this shared perception of reduced harm remained central.
How ARAC Conducts TPPI on Nicotine Pouches Under FDA Compliance
The study, conducted in late 2023 (October-November), involved 1,423 U.S. adults, including 432 smokers and 991 non-smokers. Participants were recruited online through a screening process to ensure they met specific inclusion criteria. The study focused on adults aged 21 to 65.
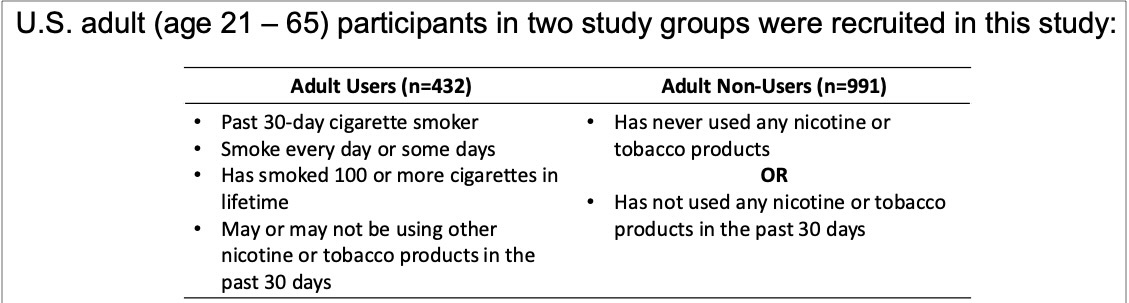
Three types of oral nicotine white pouches (ONWP) were tested: a general variant, a flavored version, and a tobacco-flavored option. Participants were shown product stimuli before being asked about their intentions to try, use, or switch to the product, as well as their perceptions of the associated risks.
The ARAC study was designed to explore two key dimensions:
- Comparing Tobacco Users and Non-Users: The study examined how tobacco users and non-users viewed ONWP products, focusing on their intentions to try and use the product and their risk perceptions.
- Intentions vs. Risk Attitudes: The study assessed both participants' behavioral intentions—whether they would try or continue using ONWP—and their attitudes toward the perceived risks of using such products.
The Study's Impact: Dissecting Misconceptions, Informing FDA Policy, and Guiding Industry Innovation
The findings of ARAC's TPPI study represent a pivotal moment in how nicotine pouches are perceived, especially in relation to their appeal to non-smokers. By addressing long-held misconceptions, the research offers a clearer understanding of the role these products might play in harm reduction and public health.
The study offers key insights for regulatory decision-making, demonstrating that ONWPs could significantly reduce health risks for smokers while posing minimal risks to non-smokers. This finding aligns with the FDA's Appropriate for the Protection of Public Health (APPH) standard, suggesting that ONWPs may meet the criteria for approval under the PMTA.
The study shows that nicotine pouches align with FDA standards, suggesting a quicker shift toward compliance. This could reduce gray-market and counterfeit products, creating a more transparent, regulated market. Companies committed to compliance stand to benefit. Additionally, the finding that flavored pouches are less appealing to non-smokers than tobacco flavors could spur innovation in flavor development, provided it is scientifically validated and meets regulatory standards.
Study Limitation: The Lack of Adolescent Data
A limitation of this study is its exclusive focus on adult participants, leaving out a crucial demographic: adolescents. Flavors in tobacco products are widely believed to attract younger users. However, the study’s conclusions—showing that flavors have a weaker appeal for non-smokers compared to smokers—are based solely on adult data. This creates a significant gap, as adolescents, influenced by social and developmental factors, may respond differently to flavored products.
ARAC BIO
Applied Research and Analysis Company (ARAC) is a leading U.S. based behavioral science research firm that designs, executes, and presents scientifically-sound, yet customizable studies to support manufacturers, regulatory agencies, and industry consultants. Their expertise aincludes consumer-focused research services in product development and innovation and regulatory science supporting marketing authorization applications, with SUCCESS in U.S. product authorizations and applications.
ARAC specializes in MODULE 5 & 6 studies including: label/claim development and comprehension, human factors/usability testing, and clinical/behavioral studies, such as randomized experimental longitudinal, actual use, TPPI, and post-market surveillance systems.
"Most Outstanding Service to Industry" 2024 Golden Leaf award-winning fully staffed IN-HOUSE psychologists, behavioral scientists, statisticians, survey methodologists, and medical monitoring offer tailored research solutions with unparalleled integrity and an exceptional client experience. (Source: ARAC)
2Firsts is dedicated to reporting on global THR scientific research and fostering deeper and more comprehensive exchanges among science, industry, and regulation. Our mission is to advance the global development of THR initiatives.
We welcome article submissions, interview opportunities, or commentary. Please contact us at info@2firsts.com or connect with 2Firsts CEO Alan Zhao on LinkedIn here.







