
In recent months, as the issue of adolescent e-cigarette use has become increasingly prominent and the regulatory efforts of the US Food and Drug Administration (FDA) have intensified, many new tobacco companies are attempting to introduce products equipped with age-gating technology to comply with regulations. According to Smoore International's 2024 financial report, the company has assisted "strategic clients" in submitting pre-market tobacco product applications (PMTA) to the FDA for multiple flavored electronic vapor products with age verification features. The core of this trend lies in using technology to isolate underage users and balance the dual pressures of compliance and market demand.
Feasibility of age verification technology
Firstly, for a long time, flavored e-cigarettes (such as fruit flavors or candy flavors) have been controversial due to their potential attractiveness to minors. These products are considered to potentially lure young people into trying e-cigarettes and lead to nicotine dependence. Therefore, when reviewing e-cigarette products, the FDA prioritizes age verification features, believing that they can effectively reduce the risk of youth exposure.
Furthermore, according to the core logic cited by the FDA in section 910 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), the approval of a product depends on whether it meets the standard of being "Appropriate for the Protection of Public Health" (APPH). Non-tobacco flavored e-cigarettes must demonstrate, compared to tobacco flavored products, that they provide an "incremental benefit" to adult smokers. This assessment must be supported by scientific evidence such as randomized controlled trials (RCT) or longitudinal cohort studies. (Analysis of FDA Regulatory Science Policy Memo: Age-verification products receive priority review, non-tobacco flavors must demonstrate "incremental benefit")
Age verification technology can help address these two issues and serve as a bridge between regulatory requirements and the actual application of products.
On one hand, strict age verification at the point of sale can significantly reduce the likelihood of minors obtaining various e-cigarette products, including those with fruit and candy flavors. On the other hand, for non-tobacco flavored e-cigarettes, reliable age verification mechanisms can help companies collect and analyze data on their actual user demographics. If data shows that, under strict age verification measures, the main users of non-tobacco flavored e-cigarettes are still adult smokers, this can provide strong evidence to support the argument for the product's "incremental benefits.
Industry giants are vying for strategic positioning
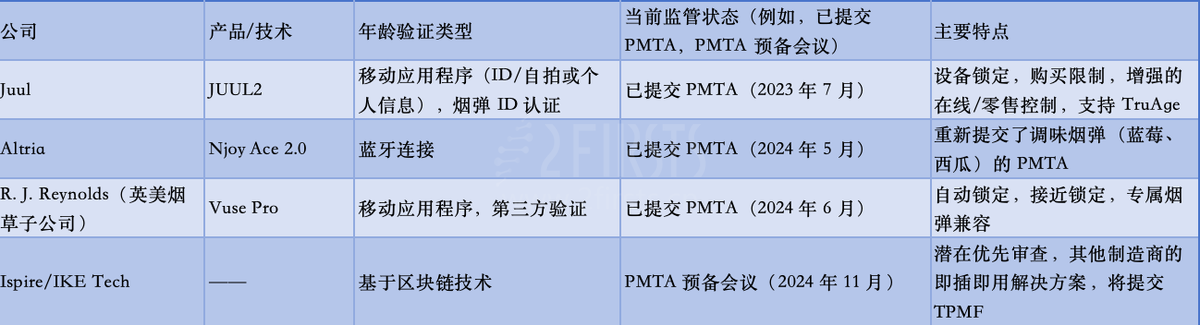
In response to regulatory pressure, major e-cigarette companies are actively developing and implementing more advanced age verification solutions. Here are the latest developments from the industry's leading companies gathered by 2Firsts.
Juul Labs announced on July 19, 2023, that they are seeking authorization in the United States to produce a new type of e-cigarette. This e-cigarette features age verification technology to prevent the use of unauthorized refill pods. It also includes a unique ID chip to prevent the use of counterfeit cartridges and comes with an app that restricts use by minors.
Altria: On May 20, 2024, Altria's subsidiary NJOY announced that it has submitted a pre-market tobacco product application (PMTA) to the Food and Drug Administration (FDA) to commercialize and sell the NJOY ACE 2.0 device. The new device features access restriction technology aimed at preventing underage use by connecting via Bluetooth and requiring user authentication before unlocking the device. The company also resubmitted PMTAs for blueberry and watermelon pod products designed to be used specifically with the NJOY ACE 2.0 device.
R. J. Reynolds: On June 27, 2024, Reynolds Tobacco announced that it has submitted a PMTA application to the FDA. The Vuse Pro device utilizes technology that unlocks after verifying that the user is at least 21 years old and above the legal minimum purchasing age for tobacco and vaping products; the Vuse Pro device is used in conjunction with Vuse Pro pods. As part of the submission, Reynolds provided the FDA with nearly 80,000 pages of scientific data for review, including 97 scientific studies.
Ispire Technology: On November 18, 2024, Ispire and IKE Tech announced that they held a pre-market tobacco product application (PMTA) submission meeting with the FDA Center for Tobacco Products (CTP). They introduced their age verification technology, which is designed to allow adults to use e-cigarettes while preventing youth access to such products. IKE Tech submitted a PMTA application, and if approved, e-cigarette manufacturers can incorporate their blockchain-based age restriction solution. The FDA indicated that they may prioritize the review and acceptance of PMTA applications.
Currently, the driving force behind businesses investing in age verification technology remains tightened regulations. In 2019, the federal Tobacco 21 law in the United States raised the minimum age for purchasing tobacco products to 21. In September 2024, the FDA will further implement new rules requiring retailers to ID customers under 30 when buying tobacco products, with some states even enacting stricter local regulations.
Based on this, the widespread application of age verification technology is not only an effective means to address the issue of teenage use, but also an important way to meet regulatory requirements and retain flavored products for adult consumers. Judging from the investments of major tobacco companies, age verification technology is becoming the "entry ticket" for the e-cigarette industry.
As U.S. regulatory policies continue to evolve and technology advances, finding a balance between compliance and innovation will remain a key focus for both the industry and regulatory agencies.
Notice
1. This article is provided exclusively for professional research purposes related to industry, technology and policy. Any reference to brands or products is made solely for the purpose of objective description and does not constitute an endorsement, recommendation, or promotion of any brand or product.
2. The use of nicotine products, including but not limited to cigarettes, e-cigarettes, and heated tobacco products, is associated with significant health risks. Users are required to comply with all relevant laws and regulations in their respective jurisdictions.
3. This article is strictly restricted from being accessed or viewed by individuals under the legal age.
Copyright
This article is either an original work by 2Firsts or a reproduction from third-party sources with the original source clearly indicated. The copyright and usage rights of this article belong to 2Firsts or the original source. Unauthorized reproduction, distribution, or any other unauthorized use of this article by any entity or individual is strictly prohibited. Violators will be held legally responsible. For copyright-related matters, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have utilized AI to enhance translation and editing efficiency. However, due to technical limitations, errors may occur. Readers are advised to refer to the sources provided for more accurate information.
This article should not be used as a basis for any investment decisions or advice, and 2Firsts assumes no direct or indirect liability for any errors in the content.










