Key Points:
·The well-known American e-cigarette retailers DEMANDVAPE and MIDWEST DISTRIBUTION have implemented a registration system that is only open to corporate users.
·DemandVape platform adds warning statement, including nicotine health risks, battery safety, etc.; at the same time, declaring that website prices are base prices, and prices may increase after selecting certain configurations.
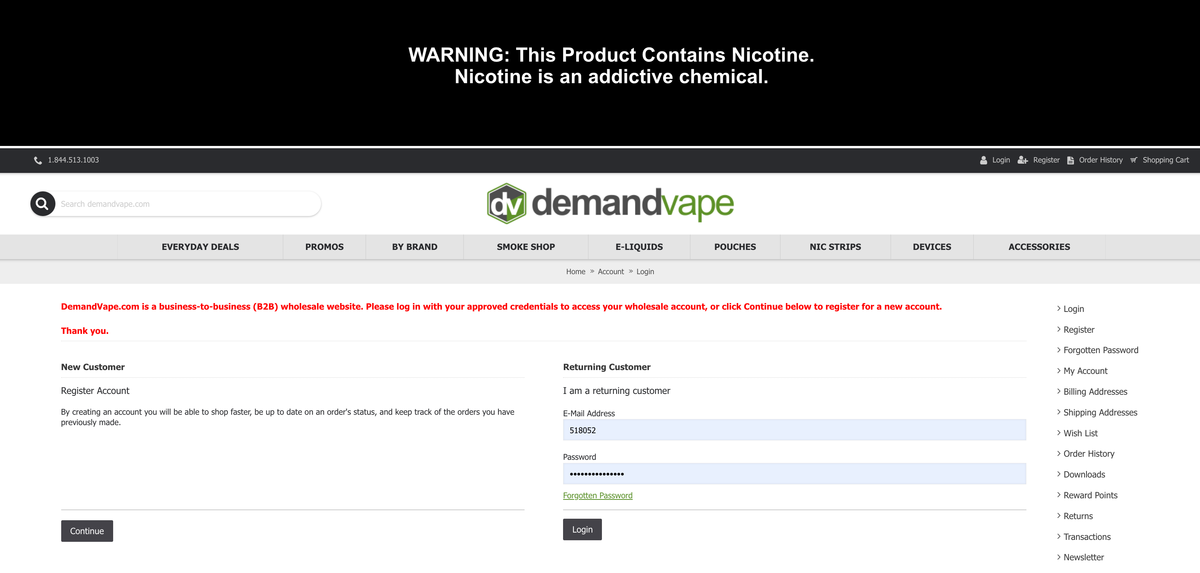
【By 2Firsts】On May 22, 2Firsts noticed that the well-known American e-cigarette distribution platform DEMANDVAPE has officially launched a registration system and explicitly stated that it is only open to enterprise users.
On its official website homepage, DEMANDVAPE prominently states in bold red font:
"DemandVape.com is a business-to-business (B2B) wholesale website. Please log in with your approved credentials to access your wholesale account, or click on the 'Continue' button below to register for a new account."
It is worth noting that before this, DEMANDVAPE was a platform open to all users, allowing them to browse all product information without the need for registration.
Meanwhile, DEMANDVAPE has added a longer warning statement to the bottom of its homepage, addressing health risks associated with nicotine use, battery safety tips, and user responsibilities:
"Warning: This product is intended for use only by individuals aged 21 and older. Children, pregnant or breastfeeding women, individuals with heart disease, high blood pressure, diabetes, those at risk, or currently taking antidepressants or asthma medication should not use this product. If you have allergies or sensitivities to nicotine or any inhalable ingredients, consult a doctor before use. This product is for recreational use only and is not a smoking cessation product, nor has it been tested for smoking cessation efficacy. Nicotine is highly addictive, so keep this product out of reach of children. This product contains nicotine, which has been classified by the state of California as a harmful substance that may cause birth defects or other reproductive harm."

In addition, the warning also detailed the potential risks of lithium batteries and put forward a number of safety operation suggestions, including avoiding the use of unapproved charging equipment, not charging unattended, using fireproof containers, and avoiding mixing battery brands and models. The warning pointed out that improper operation could lead to overheating, explosions, personal injury, or even death.
Finally, DEMANDVAPE reminds customers that the prices listed on the website are base prices, and prices may slightly increase after selecting certain configurations.
It is worth mentioning that 2Firsts also discovered that another well-known American e-cigarette distributor website, MIDWEST DISTRIBUTION, has also implemented a registration system, restricting access to only approved corporate clients.

2Firsts’ Develop PMTA Solution for U.S. Vaping Market
PMTA SUPER SERVICE PLAN
10 SKUs. One stop PMTA service.
Why pay $100K for one PMTA?
We cover ten — with better strategy, faster delivery, and global expertise.
Acceptance Pro Solution – $20,000 for 10 SKUs
Fast-track to FDA Acceptance Letter in 2–3 months
Ideal for U.S. market entry and state directory registration
SciAsset Filing Plan – $50,000 for 10 SKUs
Enter scientific review. Build long-term regulatory value
Backed by 2Firsts’ exclusive scientific compliance framework
Contact:
Michelle Zhang
E-mail: zhangmixuan@2firsts.com
WhatsApp: +79099950122
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







