Key Points:
- Compliance Dividend: BAT reports a "material improvement" in Vuse’s H2 performance, explicitly crediting intensified U.S. enforcement for clearing shelf space of illicit rivals.
- The ‘54%’ Vacuum: An isolated FDA figure suggests the illicit market share may have temporarily dipped from >85% to 54% during the Feb-Sept enforcement cycle, creating the revenue window Vuse exploited.
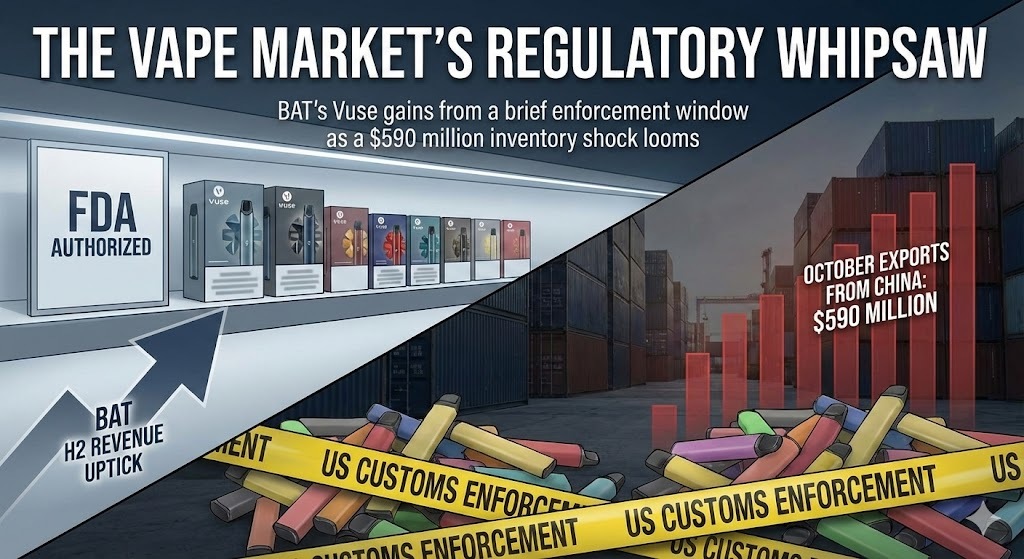
- Inventory Shock: The respite appears fragile; October vape exports from China to the U.S. surged to a record $590 million, signaling a "bullwhip" resurgence of gray market inventory.
- Strategic Defense: Doubling down on compliance, BAT launched a £1.3bn buyback while tactically pausing the rollout of its own unapproved 'Vuse One' disposables to fortify its regulatory standing.
2Firsts,Dec. 9, 2025,After years of battling a flood of unauthorized competition, the "compliance dividend" in the U.S. e-cigarette market appears to be finally materializing for incumbent players.

British American Tobacco Plc (BAT) signaled a pivotal shift in its FY2025 Pre-Close Trading Update released today, reporting that its flagship vapor brand, Vuse, delivered a "material improvement" in revenue and volume during the second half of the year. Chief Executive Officer Tadeu Marroco explicitly attributed this turnaround—a reversal of double-digit declines seen in the first half—to a shifting regulatory landscape, citing "federal and state enforcement actions" as the catalyst for clearing illicit inventory.
This is more than standard corporate optimism. When triangulating isolated FDA data with recent supply chain intelligence, BAT’s resurgence appears to be the result of a precise strategic capture of a regulatory "time window": a period where enforcement effectively, albeit temporarily, compressed the gray market.
The ‘54%’ Signal: Evidence of a Temporary Vacuum
The groundwork for BAT’s H2 improvement may have been laid two months ago in a largely overlooked regulatory disclosure.
On September 30, 2025, FDA Commissioner Marty Makary issued a statement urging retailer compliance, citing a specific, isolated figure: "As much as 54% of vaping products sold nationally are illegal."
The figure raised eyebrows among industry analysts. For years, the consensus estimate among federal agencies and third-party trackers has pegged the illicit market share at 85% or higher.
While the FDA did not provide granular sourcing for the data, market observers speculate that this lower 54% figure likely reflects a temporary trough achieved during a high-intensity enforcement cycle between February and September 2025. During this period, Customs and Border Protection (CBP) and the FDA ramped up interdiction efforts.
If this hypothesis holds, the enforcement actions successfully removed a substantial portion of unauthorized inventory—potentially creating a market vacuum. It was in this window that Vuse, armed with its Marketing Granted Orders (MGOs), broke through the structural suppression of the gray market to capture compliant demand, leading to the reported performance improvement.
The $590 Million Shock: The Bullwhip Effect Strikes Back

However, supply chain data suggests this respite may be short-lived.
According to a recent special report by the e-cigarette industry monitor 2Firsts, titled “China’s Vape Exports to the U.S. Hit a Record $590 Million: A Peak Driven by Enforcement Cycles, Not Real Demand,” the market is facing a new wave of inventory overhang.
Citing official Chinese customs data, the report highlights that in October 2025—just one month after the FDA’s statement—vape exports to the U.S. surged to a record $590 million.
Analysts interpret this not as a spike in consumer demand, but as a classic supply chain "Bullwhip Effect." Following months of tight border controls, distributors likely engaged in retaliatory restocking once enforcement pressure appeared to ease in October. While the specific drivers for the October clearance facilitation remain debated—with some observers pointing to potential resource diversion during the U.S. government shutdown scare as a contributing factor—it is likely a confluence of complex variables. The outcome, however, is clear: a massive volume of inventory has entered the channel.
This suggests that while BAT capitalized on the Q3 "cleanup," a fresh wave of unauthorized products may reignite price wars and market share dilution in early 2026.
The Strategic Wager: Fortifying the ‘Compliance Moat’
Facing this volatility, BAT is doubling down on its capital discipline and compliance narrative.
The company announced a £1.3 billion (approx. $1.7 billion) share buyback program today, signaling confidence in its ability to navigate the regulatory chop. The core thesis remains that regulatory tightening is a secular trend, and Vuse’s MGO status provides a permanent license to operate that competitors lack.
BAT is also maneuvering tactically to protect this regulatory standing. As reported by Reuters, the company paused the U.S. rollout of Vuse One in October.
According to industry data, Vuse One is the rebranded iteration of Pacha, a disposable line BAT acquired. The product line includes 17 flavored SKUs currently pending Pre-Market Tobacco Product Application (PMTA) review. By halting the expansion of a product sitting in the regulatory "grey zone" while simultaneously calling for stricter enforcement against illicit vapes, BAT appears to be fortifying its compliance profile to avoid regulatory hypocrisy during this sensitive period.
Outlook: A Fragile Victory in a Long War
The second half of 2025 provided a "sweet spot" for compliant majors like BAT. The company proved that when the enforcement machinery functions, its authorized portfolio can rapidly convert market share into revenue.
However, the record $590 million import surge in October serves as a stark warning. The structural war between federal regulators and the agile, unauthorized supply chain is far from over. Vuse’s ability to sustain its recovery in 2026 will depend entirely on whether the FDA can close the enforcement gap before the new wave of inventory floods retail counters.
Illustration created by AI
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.



