
Key Points
- Social media users claim to have purchased Altria’s new on!PLUS nicotine pouches in the U.S.
- 2Firsts found the on! website had enabled online purchases and a store locator.
- on!PLUS is produced by Altria’s subsidiary Helix and has not been approved by the FDA.
- Around the same time, BAT suspended the U.S. launch of its unlicensed Vuse One vape product.
2Firsts, October 28, 2025 — A user on social platform X (formerly Twitter) recently posted that they successfully purchased on!PLUS, a new nicotine pouch product from Altria Group’s (Altria) on! brand, within the United States. 2Firsts confirmed that the brand’s official website previously allowed online purchases and in-store location searches.
When 2Firsts visited the official shopping website onnicotine.com, two products were displayed — the on! (square box type) and on!PLUS — with a notice indicating that purchases were available online, though a strict age verification and registration process was required.

On the “Find a Store” page, users could enter a U.S. ZIP code to locate nearby retail outlets carrying the products. When 2Firsts entered the ZIP code “90001” for South Los Angeles, the system displayed around 50 store locations, most of which were 7-Eleven convenience stores.
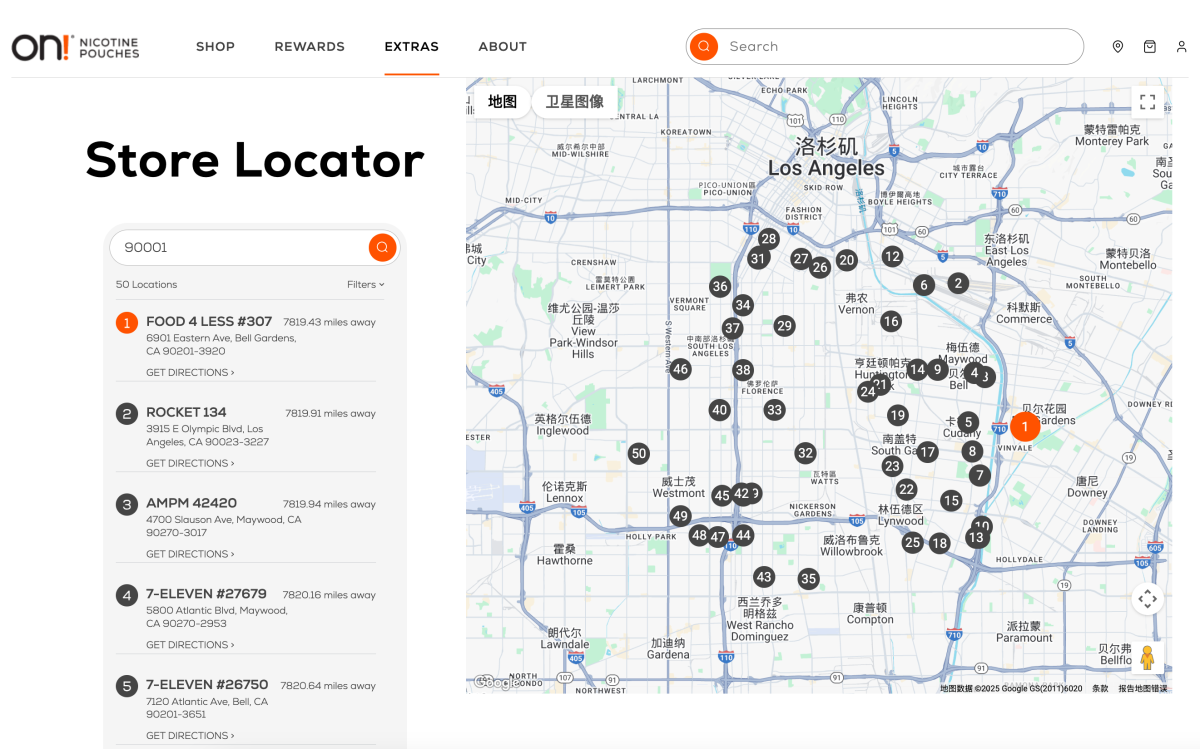
The user who shared the post wrote: “Credit where it's due: fast shipping and UPS driver made sure an adult received the package.”

Background: Proceeding Despite Lack of FDA Authorization
Altria previously stated that despite not yet receiving authorization from the U.S. Food and Drug Administration (FDA), it still plans to proceed with the on!PLUS market rollout in compliance with regulatory requirements.
(Related Reading:Altria’s Nicotine Pouch Rollout in the U.S.: On! PLUS May Launch on October 14, Not Yet FDA-Approved)
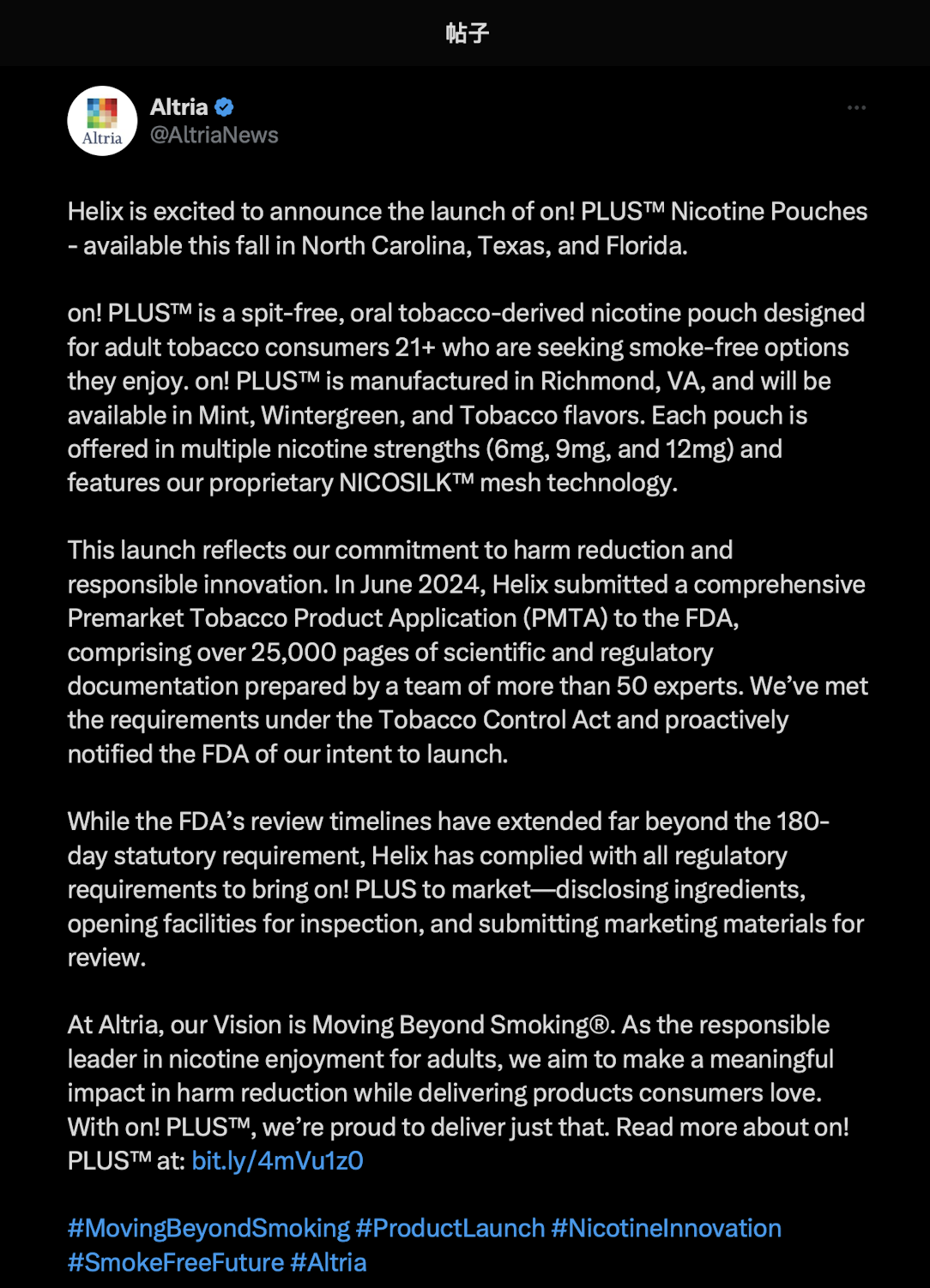
According to discussions in the Reddit community r/NicotinePouch in mid-October, several users said they had received launch notification emails from the on! brand. Initial flavors reportedly included Mint, Wintergreen, and Tobacco, with the first release targeting North Carolina, Texas, and Florida.
on!PLUS is manufactured and distributed by Helix Innovations, an Altria subsidiary. Helix submitted a Premarket Tobacco Product Application (PMTA) exceeding 25,000 pages to the FDA in June 2024, covering scientific data and compliance documentation.
Regulatory Context: BAT Suspends Unlicensed Product Launch
In late August, Reuters reported that British American Tobacco (BAT) planned to pilot its disposable synthetic nicotine vape Vuse One in several U.S. states, including South Carolina, Florida, and Georgia — a product also lacking FDA authorization.
However, on October 28, Reuters published an update stating that BAT had paused the pilot launch due to enforcement actions by the FDA against unapproved nicotine products.
Industry observers note that Altria’s on!PLUS rollout and BAT’s simultaneous suspension illustrate the tightening regulatory climate facing the U.S. nicotine product market.
(Related Reading:BAT Pauses U.S. Launch of Unlicensed Vuse One Vape amid FDA Crackdown — Reuters)
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








