
According to a report by Oaltoacre on June 14th, an investigation by the Brazilian Ministry of Justice and Ministry of Public Security found approximately 870 links offering e-cigarette products without authorization from Anvisa.
In April, the Brazilian National Health Surveillance Agency (Anvisa) passed a resolution to continue the ban on the sale of e-cigarettes in Brazil. This means that the product's sale, manufacture, import, transport, storage, and advertising are still prohibited. Five directors voted to continue the ban that has been in place in Brazil since 2009. However, the reality is different.

The Brazilian Ministry of Justice has released the results of an investigation entitled "Illegal Tobacco Products and E-cigarette Devices (DEF) Sales Online" which reveals approximately 870 websites suspected of selling tobacco products, e-cigarettes, and accessories illegally. Additionally, searching through Google, it was discovered that there are 305 public Instagram accounts involved in the same activities. Manual investigations in five states and 945 cities found 298 stores using iFood services to sell tobacco products and e-cigarette related items.
Furthermore, the survey estimated tobacco industry consumption among the population aged 12 to 65 in Brazil. Approximately 51 million people have ever smoked (33.5% of the population), with about 20.8 million people having smoked in the 30 days prior to the survey, making up 13.6% of this age group.
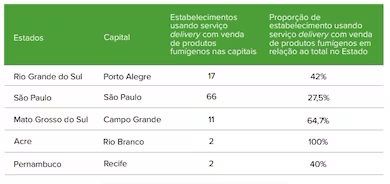
The survey evaluated 945 cities in 5 states that were surveyed, with the following results:
In all states involved, businesses have been found using iFood's delivery service to sell tobacco and e-cigarette related products. The majority of businesses selling tobacco and e-cigarette related products through iFood are located in the southeast and south of Brazil, followed by the central-west, and lastly the northeast and north. Compared to other cities, major urban areas have more businesses using delivery services to sell tobacco products, with a wider range of products and higher prices. Typically, businesses employ a range of strategies, such as using specific keywords to name tobacco products and e-cigarette devices, in order to circumvent control and tracking systems.
In social media platforms, sales websites, and food delivery apps, one can often observe behaviors that violate the regulations set by the Brazilian National Health Surveillance Agency.
In Brazil, it is prohibited to sell any tobacco products or e-cigarette related devices on the internet, as well as to advertise them to the public through displaying packaging images, product names, or brands.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








