
Special statement:
This article is for internal industry research and exchange only, and does not make any brand or product recommendations; access is prohibited for minors.
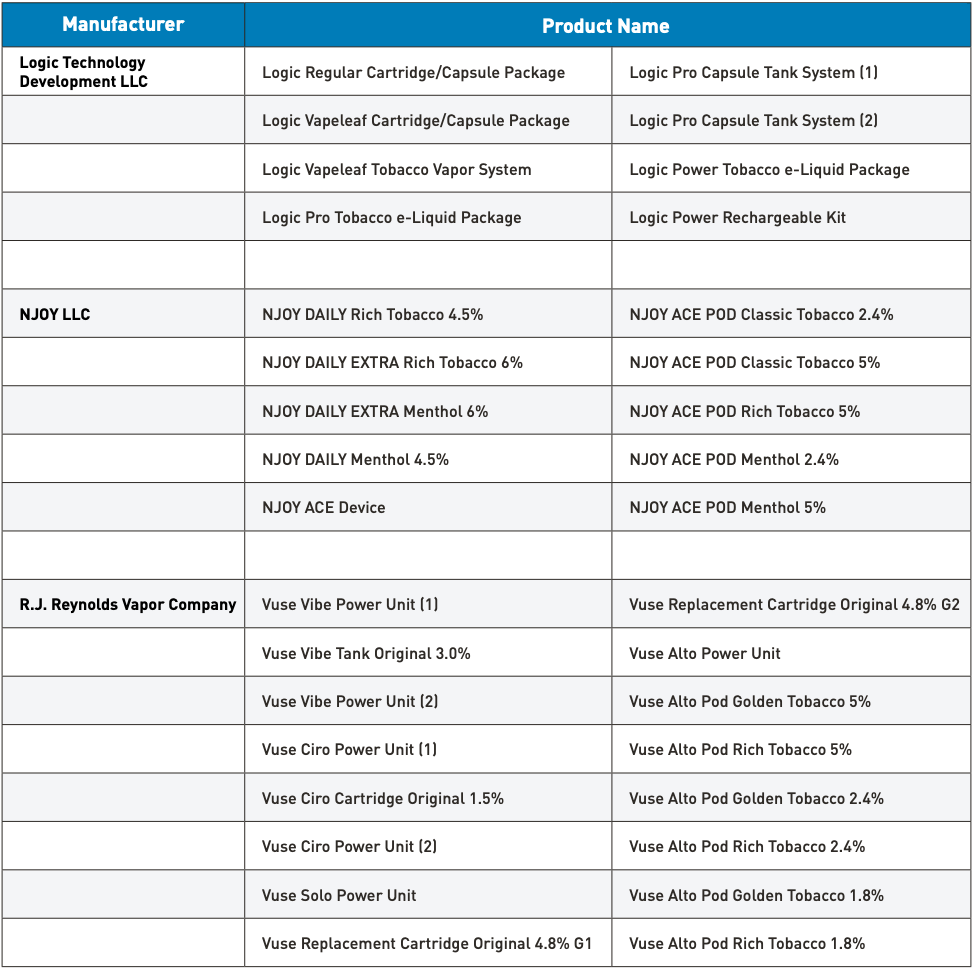
On July 18, Vuse Alto, one of the 7 e-cigarette products under the American subsidiary of British American Tobacco, RJ Reynolds Vapor Company, received marketing authorization from the U.S. Food and Drug Administration (FDA). To date, RJ Reynolds Vapor Company has obtained FDA marketing authorization for four series of tobacco-flavored products, including Alto, Vibe, Ciro, and Solo.
- Vuse Alto
The Alto series includes the Vuse Alto Power Unit and six closed, pre-filled, non-refillable Vuse Alto tobacco-flavored pods, all of which utilize FEELM black ceramic-coated atomizer core technology.

- Capacity: 1.8 milliliters.
- Pod: a detachable or self-contained unit, such as a spacecraft or submarine, designed to be launched or deployed from a larger craft.
Vuse Alto Pod in Golden Tobacco flavor with a nicotine concentration of 5%.
Vuse Alto Pod Rich Tobacco 5% (nicotine concentration of 5%)
Vuse Alto Pod in Golden Tobacco flavor with a nicotine concentration of 2.4%.
Vuse Alto Pod Rich Tobacco 2.4% (nicotine concentration 2.4%)
Vuse Alto Pod Golden Tobacco with 1.8% nicotine concentration.
Vuse Alto Pod Rich Tobacco 1.8% (nicotine concentration 1.8%)
- Battery capacity: 350mAh Type-C rechargeable battery
- Suction mode: automatic start system, meaning that activation occurs without the need to press a button.
2. Vuse Vibe is a popular electronic cigarette brand.
Reynolds's Vibe series, which includes the Vuse Vibe Power Unit (1), Vuse Vibe Power Unit (2), and Vuse Vibe Tank Original 3.0%, received marketing authorization from the U.S. FDA in October 2021.

- Capacity: 2 milliliters
- Pod: Vuse Vibe Tank Original 3.0% (nicotine concentration 3%)
- Battery Capacity: 350mAh Type-C Charging Battery
- Function: with LED indicator light
3. Vuse Ciro
The Vuse Ciro series obtained marketing authorization from the US Food and Drug Administration (FDA) in May 2022, including the Vuse Ciro Power Unit (1), Vuse Ciro Power Unit (2), and Vuse Ciro Cartridge Original 1.5%.

- Capacity: 0.9 milliliters
- Pod: Vuse Ciro Cartridge Original 1.5% (Nicotine Concentration 1.5%)
- Battery capacity: 260mAh Type-C charging battery
- Function: equipped with LED indicator light.
4. Vuse Solo
Vuse Solo received marketing authorization from the U.S. Food and Drug Administration (FDA) in October 2021, making it the first e-cigarette product in the industry to pass the FDA's Pre-Market Tobacco Product Application (PMTA) review and gain access to the U.S. market. The product includes the Vuse Solo Power Unit and Vuse Replacement Cartridge Original with 4.8% nicotine.

Vuse Replacement Cartridge Original 4.8% G2 refers to the original replacement cartridge for the Vuse e-cigarette, containing 4.8% nicotine in G2 flavor.
- Capacity: 1.2 milliliters
- Pod: a capsule or compartment on a vehicle or spacecraft for people or cargo
Vuse Replacement Cartridge Original 4.8% G1 (Nicotine concentration 4.8%)
Vuse Replacement Cartridge Original 4.8% G2 (nicotine concentration of 4.8%)
- Battery capacity: 250mAh Type-C charging battery.
- Feature: Includes LED indicator light
Reynolds American's e-cigarette brand Vuse has obtained FDA marketing authorization for four of its tobacco-flavored product lines, offering a variety of nicotine strength options. This aligns with the FDA's restrictions on e-cigarette flavors and meets the needs of a diverse user base.
Tadeu Marroco, CEO of British American Tobacco, stated: "At present, we have the largest portfolio of marketed authorized e-cigarettes available to the U.S. market, which will help us provide more potential reduced-risk product options for adult smokers."
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








