
On July 18, the official website of the Food and Drug Administration (FDA) announced the authorization of seven e-cigarette products to be sold in the United States through the pre-market tobacco product application (PMTA) pathway.
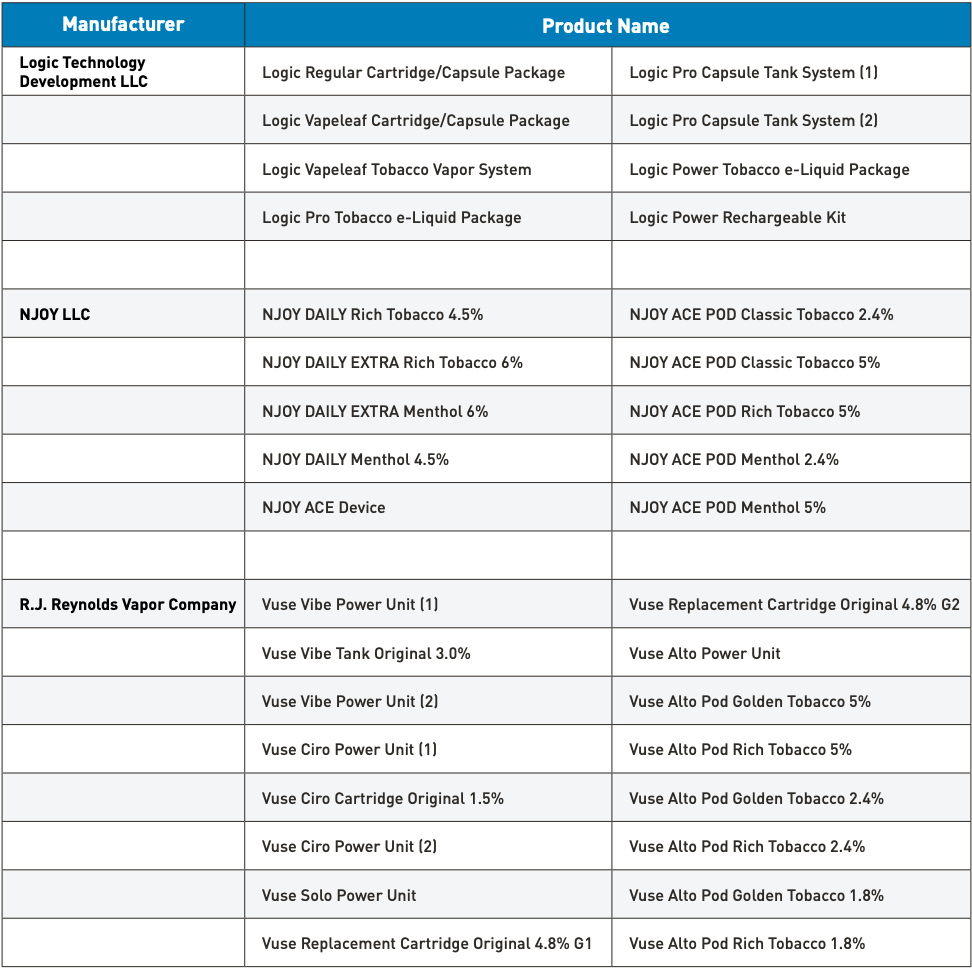
After extensive scientific review, the FDA has issued marketing authorization to R.J. Reynolds Vapor Company, involving the Vuse Alto Power Unit and six closed, pre-filled, non-refillable Vuse Alto tobacco-flavored pods.
- Vuse Alto Pod Golden Tobacco 5%
- Vuse Alto Pod Rich Tobacco 5%
- Vuse Alto Pod Golden Tobacco 2.4%
- Vuse Alto Pod Rich Tobacco 2.4%
- Vuse Alto Pod Golden Tobacco 1.8%
- Vuse Alto Pod Rich Tobacco 1.8%
The FDA emphasizes that while these tobacco products are authorized for sale in the United States, it does not mean that these tobacco products are safe or that they have been "FDA approved." Additionally, this action does not mean that these products are suitable for sale as reduced harm tobacco products. All tobacco products are harmful and potentially addictive. The FDA reminds individuals, especially young people, who do not use tobacco products, should not start using them.
The FDA evaluated PMTA based on public health standards, taking into account the risks and benefits of the products to the overall population. After reviewing the company's application, the FDA determined that there was enough evidence to suggest that allowing the sale of these products would contribute to protecting public health, as required by the 2009 Family Smoking Prevention and Tobacco Control Act. Specifically, the applicant demonstrated that these tobacco-flavored products could provide sufficient benefits to adult smokers that outweigh the risks, including risks to adolescents.
Despite FDA still concerned about the risks of all e-cigarette products for teenagers, the likelihood of teenagers using tobacco-flavored e-cigarette products is lower than other flavors. According to the 2023 National Youth Tobacco Survey, Vuse is one of the most commonly reported brands used by middle and high school students. However, only 6.4% of students currently using e-cigarettes reported using tobacco-flavored products. In order to further reduce the risk of teenagers using these products, FDA has imposed strict marketing restrictions on new products to prevent youth exposure and use, just as with previously authorized products. FDA will closely monitor the marketing of these products and will take appropriate action if companies fail to comply with any applicable laws or regulatory requirements. This could include suspending or revoking authorization if youth or former cigarette users significantly increase their use of these products, or if current cigarette users decrease to a complete transition mode to new products.
This action is one of many measures taken by the FDA to ensure that all new tobacco products sold in the United States undergo scientific review and obtain market authorization. The FDA has received nearly 27 million applications for deemed products and has made decisions on over 26 million applications.
On June 21, the FDA FDA issued marketing authorization for four mint-flavored e-cigarette products by NJOY LLC - NJOY ACE Pod Menthol 2.4%, NJOY ACE Pod Menthol 5%, NJOY DAILY Menthol 4.5% and NJOY DAILY EXTRA Menthol 6%.
So far, the FDA has authorized 34 e-cigarette products and devices, including the seven products authorized today. The FDA has compiled a printable leaflet listing all authorized e-cigarette products; these are currently the only e-cigarette products that can legally be sold and distributed. Those who produce, import, sell, or distribute e-cigarettes without premarket authorization will face enforcement risks.
As of July 2024, the only list of e-cigarette products authorized by the FDA for sale in the United States:
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








