
On December 5th, the US Food and Drug Administration (FDA) announced that it had issued warning letters to 115 retail entities for selling unauthorized e-cigarette products. The letters specifically mentioned the sale of disposable e-cigarette products produced by Chinese manufacturers and sold under well-known brand names such as Geek Bar Pulse, Geek Bar Skyview, Geek Bar Platinum, and Elf Bar.
These warning letters are part of an ongoing enforcement action coordinated by the FDA with state partners aimed at identifying and cracking down on the unauthorized sale of e-cigarettes. The FDA has contracts with states, regions, or third-party agencies to assist in inspecting retail locations for compliance.
According to the results of the 2024 National Youth Tobacco Survey, 5.8% of current adolescent e-cigarette users reported having used products from the Geek Bar brand. Furthermore, after reviewing additional rapid surveillance data and preliminary data from the Population Assessment of Tobacco and Health Study, the FDA has also confirmed that this brand has a high level of appeal to young people.
The FDA closely monitors the tobacco industry for compliance with tobacco laws and regulations under the Federal Food, Drug, and Cosmetic Act, taking action when violations are found. Businesses that receive warning letters must respond within 15 working days, outlining corrective actions taken and plans to prevent future violations. Failure to promptly correct violations may result in further action by the FDA, such as litigation, seizures, and/or civil fines.
According to regulations, new tobacco products must obtain authorization from the FDA before they can be legally sold. Generally, products that have not been authorized face enforcement risks. As of now, the FDA has authorized 34 e-cigarette products and devices. These are currently the only e-cigarette products that can be legally sold in the United States. For more information on tobacco products that can be legally sold in the United States, you can refer to the FDA's searchable tobacco product database.
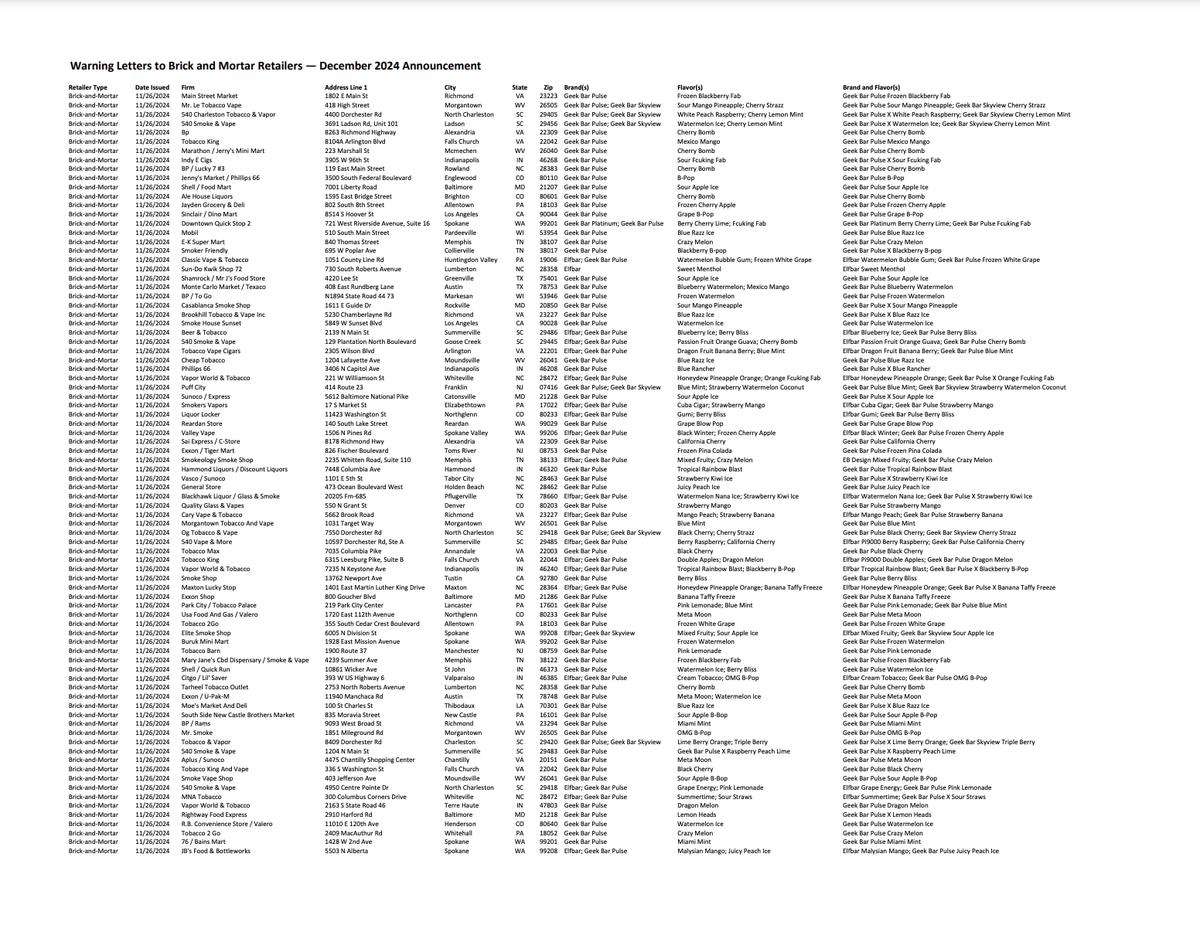
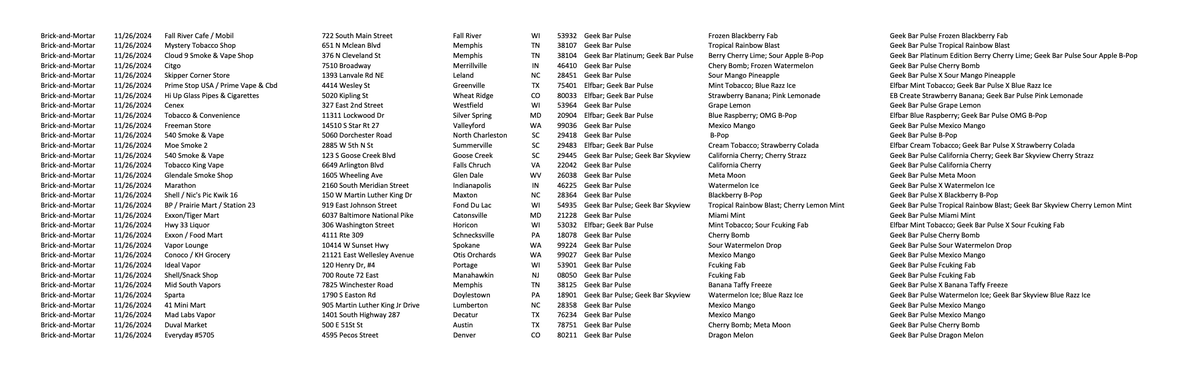
The following is the specific list:
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








