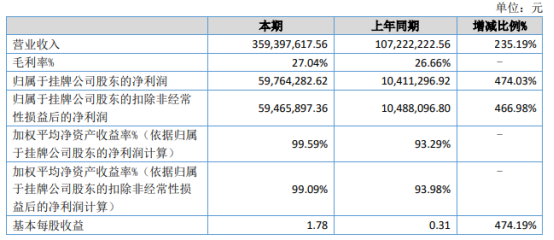
On August 16th, Wulun Technology (833767) recently released their 2022 semi-annual report. During the reporting period, the company achieved operating revenue of RMB 359,397,617.56, a year-on-year increase of 235.19%; net profit attributable to the listed company's shareholders was RMB 59,764,282.62, a year-on-year increase of 474.03%.
During the reporting period, the net cash flow generated by operating activities was RMB 84,449,058.51, with a net asset of RMB 89,895,077.97 attributable to the listed company's shareholders.
During the reporting period, the company achieved a revenue of 359,397,617.56 yuan, an increase of 235.19% year-on-year. The main reason for this is that the company's disposable electronic cigarette product design met market demand, resulting in an increase in sales of both OEM and self-branded electronic cigarette products, with the fastest growth in sales occurring in Europe.
The operating costs increased by 233.45% compared to the same period last year, primarily due to a 235.19% increase in operating revenue, resulting in a year-on-year increase in costs.
The company's operating profit increased by 548.11% compared to the same period last year, primarily due to two reasons: Firstly, this year's operating revenue increased by 235.19%, or 252,175,395.00 yuan, to 359,397,617.56 yuan, compared to 107,222,222.56 yuan in the previous period. Secondly, the company's financial expenses decreased by 669.32%, or 7,093,496.55 yuan, to -6,033,682.72 yuan, compared to 1,059,813.83 yuan in the previous period. This increase in revenue and decrease in expenses led to an increase in operating profit.
According to data from Wabei.com, Five Rings Technology's main products and services revolve around research and development, production, and sales of electronic cigarettes and related accessories.
The contents excerpted or reproduced in this article are sourced from third-party information, and their copyrights belong to the original media and authors. If there is any infringement, please contact us to delete it. Any unit or individual wishing to reproduce it must contact the author and not directly reproduce it.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.







