
Disclaimer
- This article is intended solely for internal industry communication and does not constitute an endorsement of any brand or product.
- The images presented in this article are for factual illustration purposes only and should not be interpreted as advertisements for any product.
- Access to this article is prohibited for minors.
Recently, 2Firsts noticed that the vape brand HAYATI has launched the Hayati® Mini Ultra vape in the UK market. The product features two 2ml pods, compliant with TPD standards, and offers approximately 1500 puffs.
Specifically, the product features of the Hayati® Mini Ultra include:
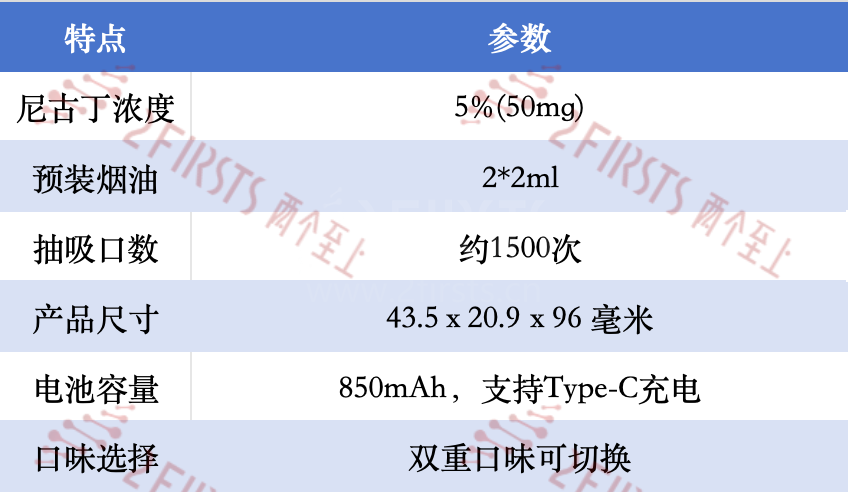
The main feature of the Hayati® Mini Ultra is its ability to hold two 2ml transparent e-liquid pods with different flavours, allowing users to switch between the two flavours by twisting the mouthpiece.

Previously, the HAYATI brand launched the Hayati® PRO Ultra vape, which also features a similar twist mouthpiece design. However, this product is equipped with two 12ml e-liquid pods and claims to provide approximately 15,000 puffs.

It is worth noting that the Hayati® Mini Ultra does not feature the electronic screen used in the Hayati® PRO Ultra for direct battery level display. Instead, it uses a color-changing "lotus-shaped" LED indicator to indirectly show the battery level.
Currently, the Hayati® Mini Ultra is available on the official HAYATI website and other well-known UK vape retailers like vapesourcing.uk, with the product page marked as "COMING SOON."
According to the official HAYATI website, the HAYATI brand is owned by PAX Innovations (Shenzhen) Limited. All product registration applications with the UK Medicines and Healthcare Products Regulatory Agency (MHRA) are submitted by this company. The HAYATI website also references "PAX Manufacturing" and includes a link to the corresponding website.

The PAX official website states that the company was founded in Hong Kong in 2021. Its founder, Mr. He, is an experienced expert in the e-cigarette industry, dedicated to the research and innovation of e-cigarette products.
2Firsts will continue to track the latest products and market trends in disposable vapes, offering the public accurate and authoritative industry information.
2Firsts' product column remains committed to delivering readers the latest product updates in the new tobacco field. We invite readers to submit insights and news on e-cigarette developments.
For unique perspectives or information, feel free to contact us at carrie.cai@2firsts.com or scan the QR code below.








