
Recently, 2Firsts noticed that the e-cigarette brand NEXA has launched a NEXA Ultra 50K disposable e-cigarette product, which is believed to be the e-cigarette product with the largest puff count in the American market to date, with the ability to be used up to 50,000 times.
Earlier, 2Firsts predicted that 50,000 units of disposable e-cigarette products would be launched in the US market in October this year, while conducting an inventory of new 40,000 units of disposable products. This prediction has been validated with the launch of NEXA Ultra 50K (for more details, refer to "Product Roundup: New 40,000-Puff Disposable E-Cigarettes in the US, Puff Count Race Intensifies).

Following the news of luckeevape's Super Luckee 45000 disposable e-cigarette with 45,000 puffs(Super Luckee 45000: The Largest Disposable E-Cigarette in USA)The new product boasts 50,000 puffs, 20ml e-liquid capacity, and a 3D curved screen design, marking a new phase in the escalating puff count competition in the American e-cigarette market.

Specifically, the main product features of the NEXA Ultra 50K include:
Nicotine concentration: 5% (50mg)
Number of puffs: approximately 50,000 (normal mode) /30,000 (turbo mode)
Battery capacity: 800mAh Rechargeable battery
Charging method: USB Type-C charging
E-liquid capacity: 20ml
Screen features: 3D curved screen, with light effects and battery indicator
Product flavors: 15 Product price: approximately $16.99

In terms of appearance, the NEXA Ultra 50K adopts the popular 3D curved screen design, (The Rise of 3D Curved Screen E-cigarette Products)which allows for real-time monitoring of the product's battery power. It also features a transparent e-liquid reservoir, providing a clear display of the remaining e-liquid levels.
By comparing Super Luckee 45000 with 2Firsts, it was noted that both are high-capacity e-cigarette products. Super Luckee 45000 has a 50ml e-liquid capacity and approximately 45,000 puffs, while the NEXA Ultra 50K has a smaller 20ml e-liquid capacity but offers 50,000 puffs.

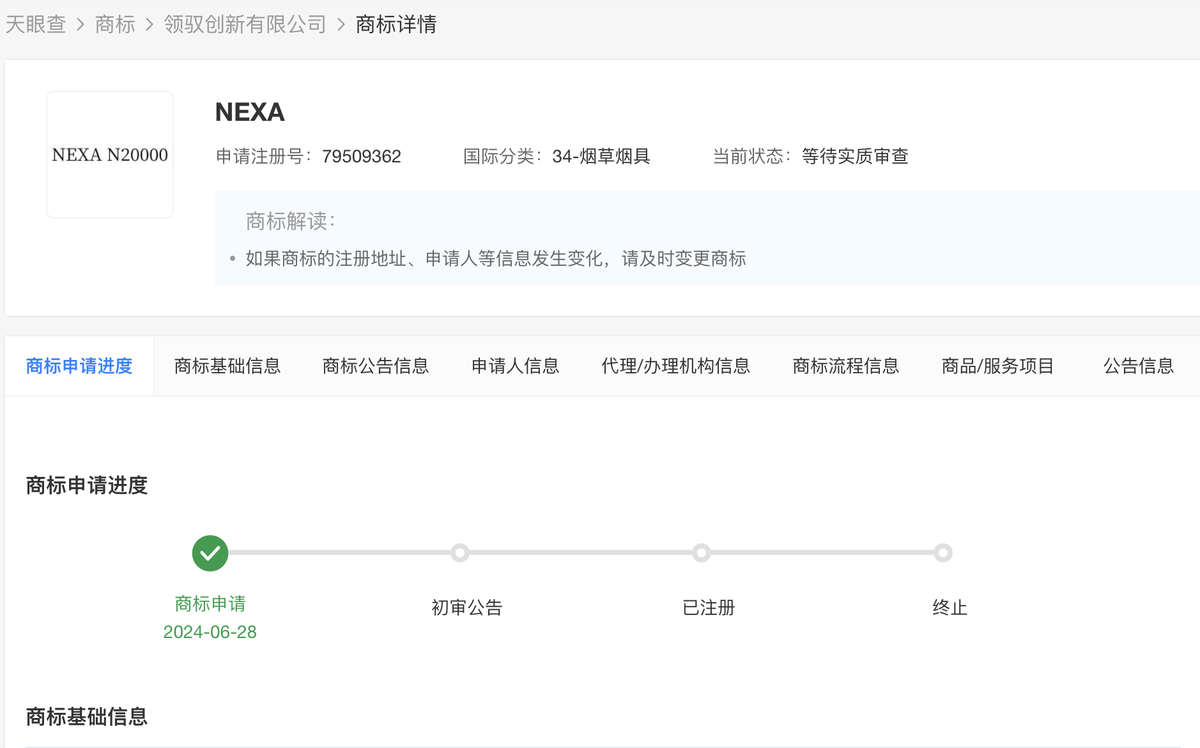
According to a survey conducted by 2Firsts, it was found that the NEXA brand trademark belongs to LingYu Innovation Limited, which was established in December 2023 and is located in Hong Kong, China. The trademark "NEXA" was officially submitted for trademark application on June 28, 2024 and is currently still awaiting substantive examination.
It is reported that the NEXA Ultra 50K has now launched on the official website of the Nexabar brand as well as on popular American e-cigarette distributor websites such as Vapesourcing and General Vape.
The product section of 2FIRSTS is dedicated to providing readers with the latest information on new products in the tobacco industry. We encourage readers to submit articles and share the latest products in the e-cigarette field with us.
If you have any unique insights or information, please feel free to contact us at carrie.cai@2firsts.com or scan the QR code below to get in touch.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








