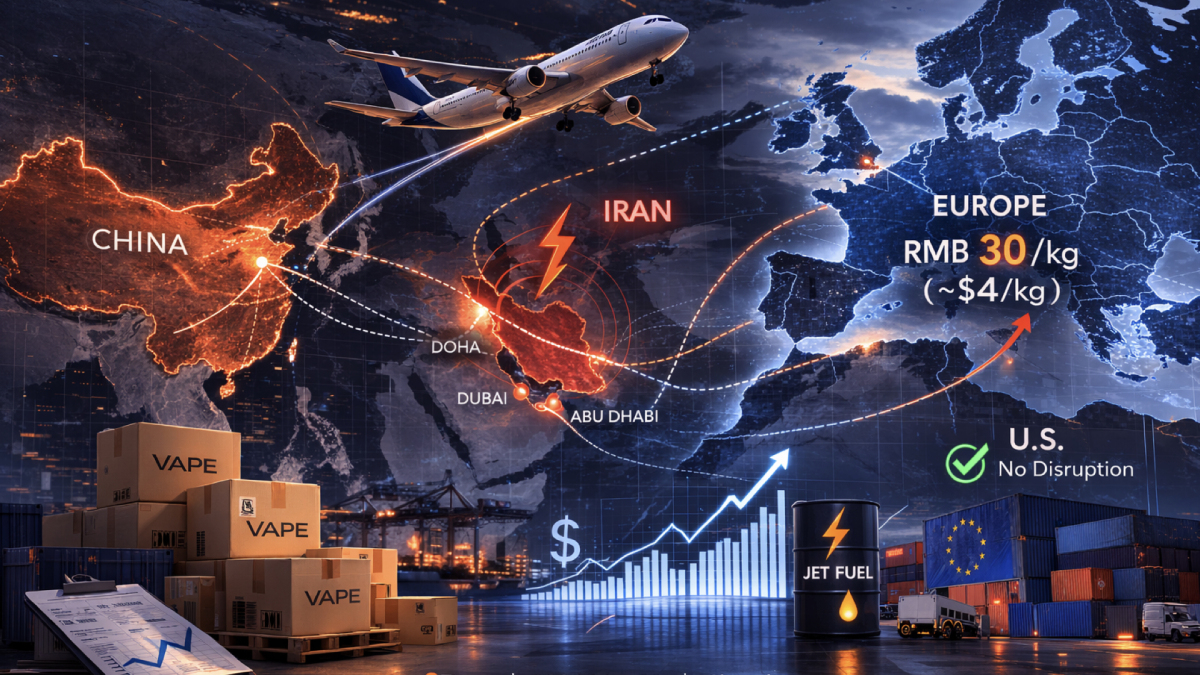
On Friday, ITC Ltd, a conglomerate in India, announced its quarterly profits which exceeded expectations due to strong cigarette sales, as well as stable demand for its packaged food products.
The business conglomerate, which operates in the tobacco and hotel industries, has announced a rise in profits for the October-December quarter. The profits have increased from 41.56 billion rupees to 50.31 billion rupees ($614 million) compared to the same period last year.
According to Refinitiv IBES, analysts predict that profits will increase to INR 47.44 billion (USD 573 million) on average.
ITC, which owns numerous brands including Sunfeast and Classmate, stated in a declaration that consumer sentiment has improved this quarter.
As commodity inflation continues to slow down, economic activity in India is gaining momentum.
ITC's overall operating revenue has increased by approximately 3%, reaching INR 172.65 billion ($2.08 billion), with over 40% of that coming from its cigarette business.
This business possesses the Classic and Gold Flake brands, with revenue growth of nearly 17% to 72.88 billion rupees.
This week in India, the cigarette industry is facing challenges in the wake of a potential 16% increase in taxes due to it being considered a national disaster. Some analysts believe that this could result in a "moderate" 1.5% increase in taxes. Others suggest that ITC should respond by raising prices.
ITC's fast-moving consumer goods (FMCG) business, which includes a combination of biscuits, noodles, snacks, and dairy products, has reported an 18% growth in revenue. The company plans to acquire Sproutlife Foods, the maker of Yoga Bars, to expand its nutrition-led health food business.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.