
Special Statement:
- This article is for internal industry communication only and does not constitute any brand or product recommendation.
- The images shown in this article are solely for factual description and do not serve as advertisements for any products.
- This article is strictly prohibited for access by minors.
Key Points:
- Brands accelerating their presence in the UK market:
- ELFBAR: Published its ELFX series and the brand-new JOINONE series devices, covering both open-system and pod-based product lines.
- VAPORESSO: Published the XROS PRO 2 KIT.
- Other brands: Including JNR, MOKI, HAYATI, OXBAR, and SMOK, also released a large number of new products.
- Significant surge in pod updates: From August 11 to 18, the MHRA published a total of 1,156 e-cigarette SKUs, among which the category “refillable pods containing e-liquid” accounted for 1,033 updates — more than triple compared to the previous week’s 319.
- Diversified product types: The newly published products cover a wide range, including rechargeable devices, refillable devices, device kits, and independent pods/components.
According to UK compliance procedures, e-cigarette products must be listed in the MHRA’s notification database. Once published, it indicates that these SKUs have passed compliance review and are legally approved for sale in the UK market.
To help industry stakeholders understand the approval status of new products in the UK market, 2FIRSTS regularly compiles and analyzes relevant information from MHRA announcements.
The following covers the announcements updated between August 11 and 17, including major brands, product types, and preliminary market trend analysis.
During this period, MHRA published a total of 1,156 SKUs, involving brands such as ELFBAR, OXBAR, MOKI, HAYATI, and JNR.
ELFBAR ELFX and JOINONE Series Updates, MOKI Launches New E-cigarette Products
In the category “Electronic cigarette – Rechargeable, device only. Any rechargeable which can also be used as a refillable should be reported under the refillable category,” a total of 14 SKUs were updated between August 11 and 17, involving brands such as ELFBAR, JNR, MOKI, HAYATI, and OXBAR.
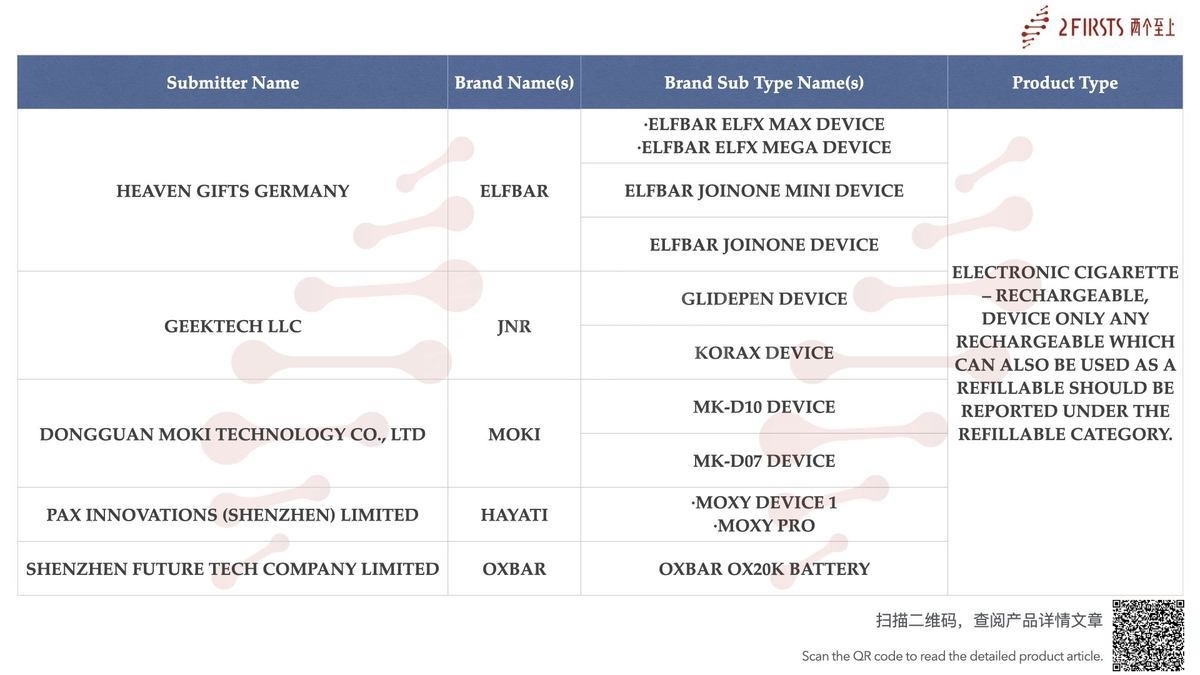
- Three ELFBAR-branded e-cigarette products submitted by HEAVEN GIFTS GERMANY — ELFBAR ELFX MAX DEVICE, ELFBAR ELFX MEGA DEVICE, ELFBAR JOINONE MINI DEVICE, and ELFBAR JOINONE DEVICE — have been published.
- Among them, the ELFX Series is an open-system series under the ELFBAR brand, which has already launched multiple products, including ELFX, ELFX Special Edition, ELFX PRO Classic Edition, ELFX PRO, and ELFX ULTRA. (Read more)
- The ELFBAR JOINONE Series is the brand’s latest pod-based e-cigarette line. It has so far introduced three pods — two Dock-to-Vape pods of different specifications and one Standalone Use pod — along with two devices: the Dynamic Device and the Lumeo Device. (Read more)

Two e-cigarette devices under GEEKTECH LLC’s JNR brand — the GLIDEPEN DEVICE and the KORAX DEVICE — have been published.
Dongguan Moki Technology Co., Ltd. has published two e-cigarette devices under its MOKI brand: the MK-D10 DEVICE and the MK-D07 DEVICE.
E-cigarette brands HAYATI and OXBAR have also published new devices — MOXY DEVICE 1 & MOXY PRO and the OXBAR OX20K BATTERY, respectively.
In addition, this product category has seen new SKU launches from other brands, including Mr.goodie, VAPMOD, LUMINOVA, FISCO, DRIPPED, and TASTEFLEX.
JNR Launches Multiple Open-System Products, HQD to Enter the UK Market
Within the category “Electronic cigarette – Refillable, placed on the market with one type of e-liquid (fixed combination)”, a total of 8 SKUs were published between August 11 and August 17, all of which belonged to the JNR brand.
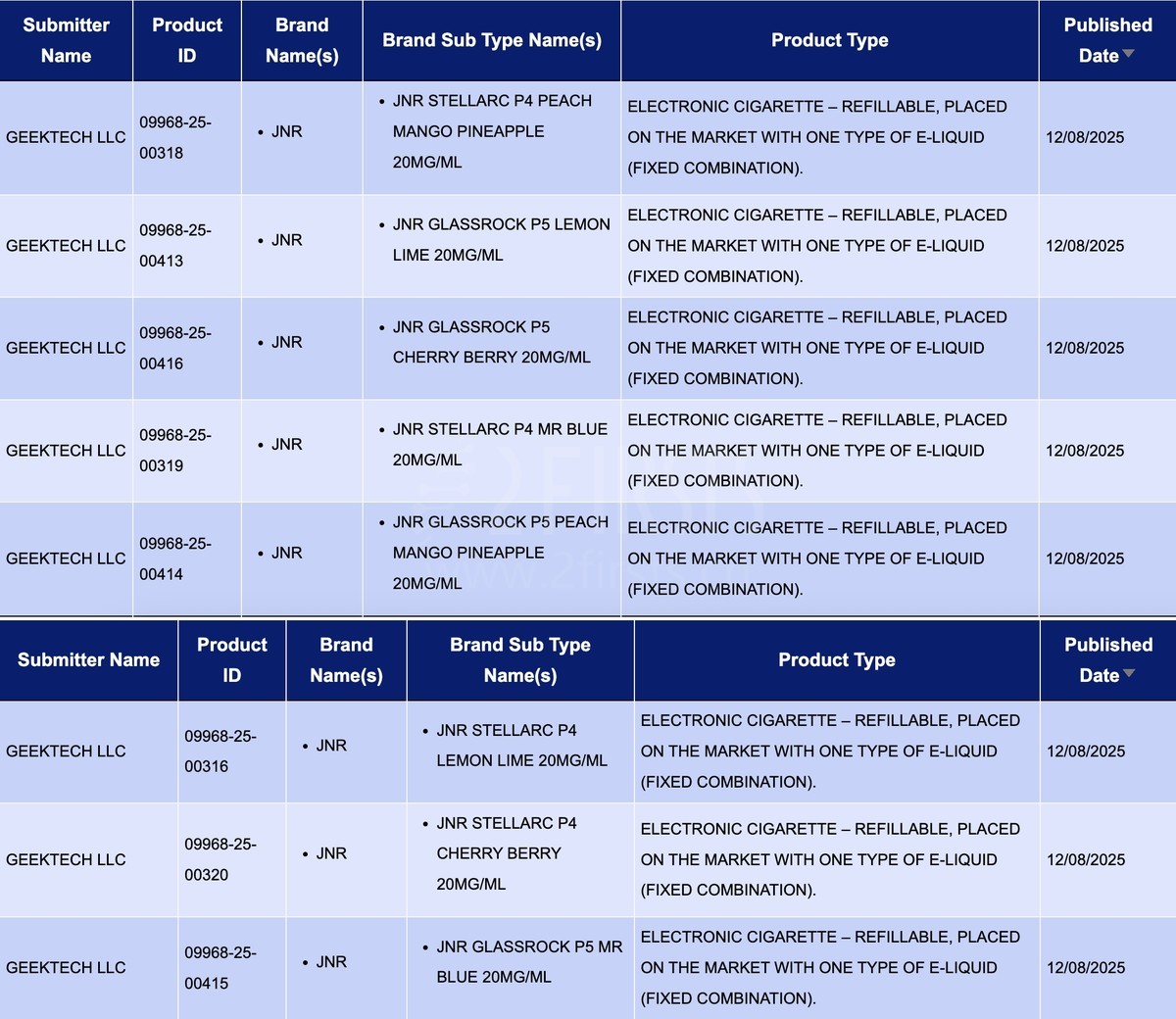
Under GEEKTECH LLC’s JNR brand, the JNR STELLARC P4 and JNR GLASSROCK P5 were each updated with four different flavored SKUs, all with a nicotine concentration of 20 mg/mL.
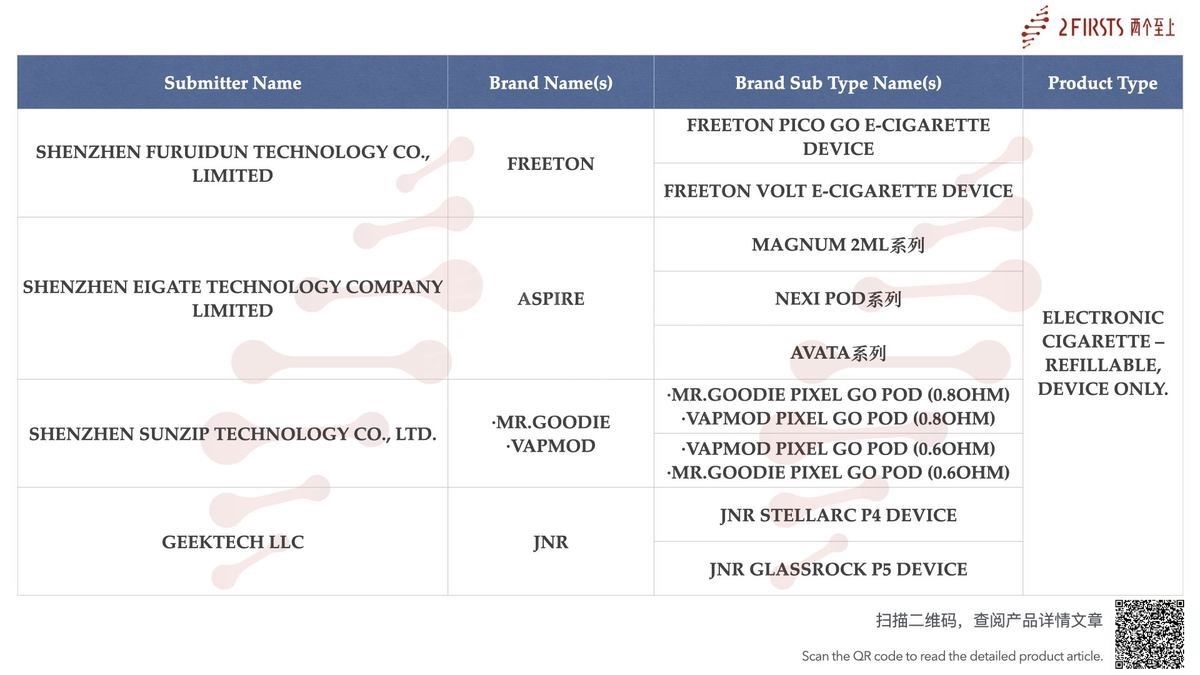
Within the category “Electronic cigarette – Refillable, device only”, a total of 27 SKUs were published between August 11 and August 17, covering brands such as HQD, OXVA, FREETON, ASPIRE, and ELEAF.
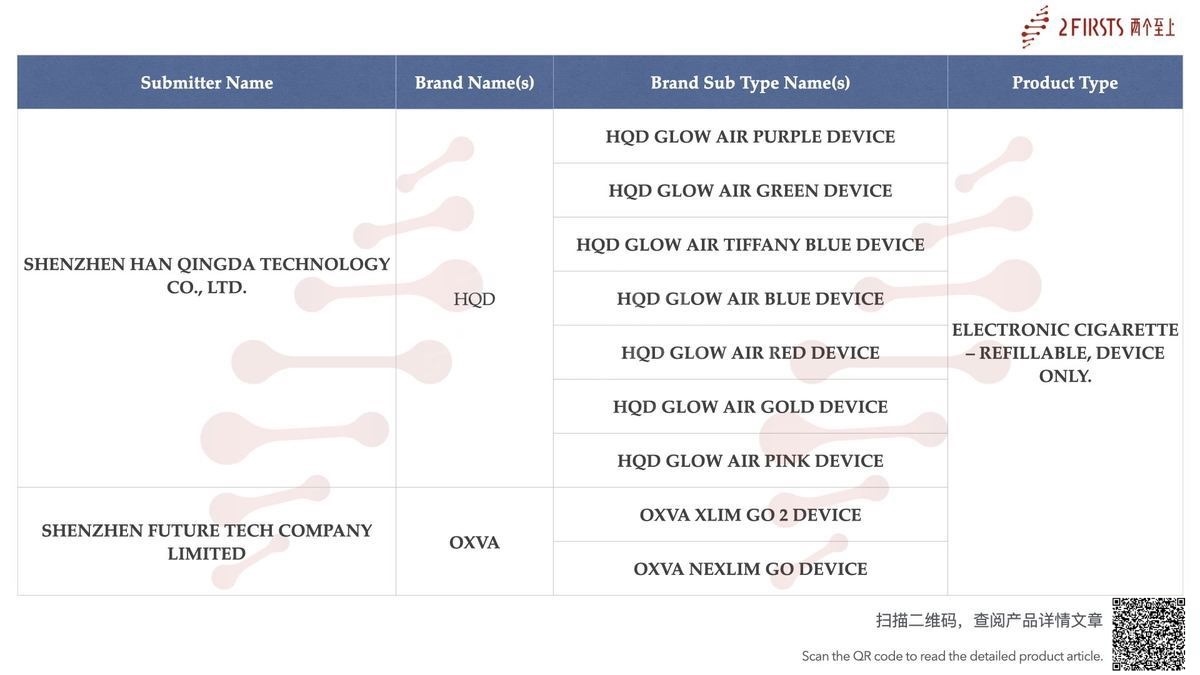
Shenzhen HAN QINGDA Technology Co., Ltd. has published new SKUs with the MHRA.
Under its HQD brand, the company introduced the HQD GLOW AIR device, which includes 8 SKUs, each corresponding to a different color. (Read more)
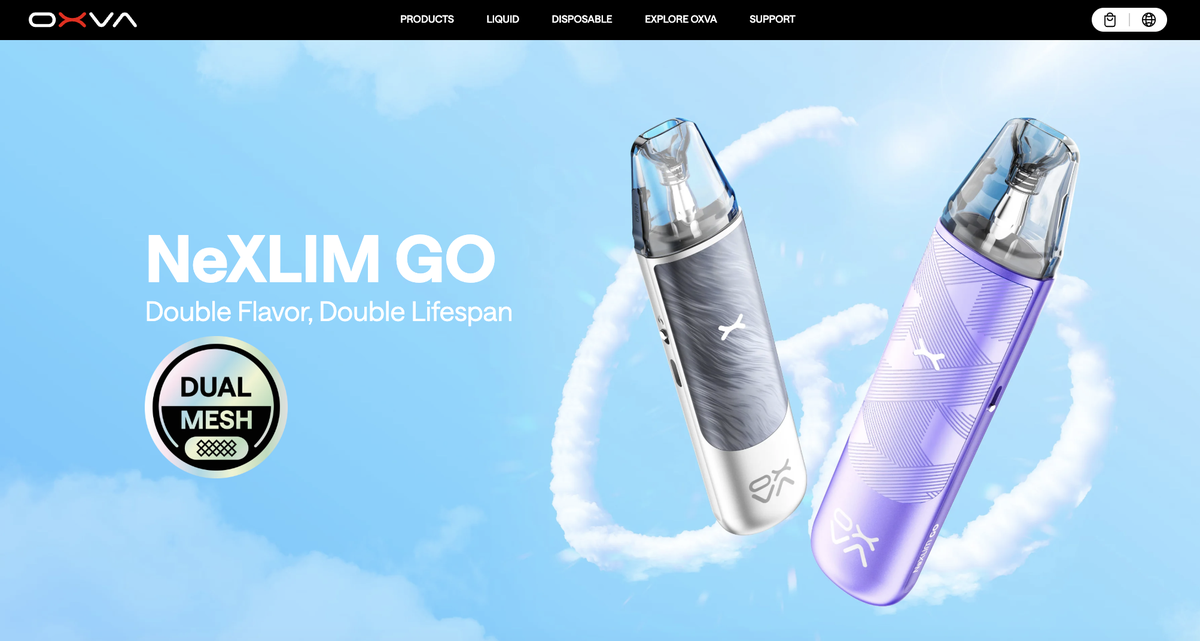
Shenzhen Future Tech Co. LTD., under its brand OXVA, has announced the public listing of two e-cigarette devices: OXVA XLIM GO 2 DEVICE and OXVA NEXLIM GO DEVICE.
Among them, the OXVA NEXLIM GO DEVICE has already been featured on the official OXVA brand website.
Other products publicly listed under this category are as follows:
VAPORESSO Updates Multiple Devices & Pods — Pod Listings Triple Compared to Previous Period
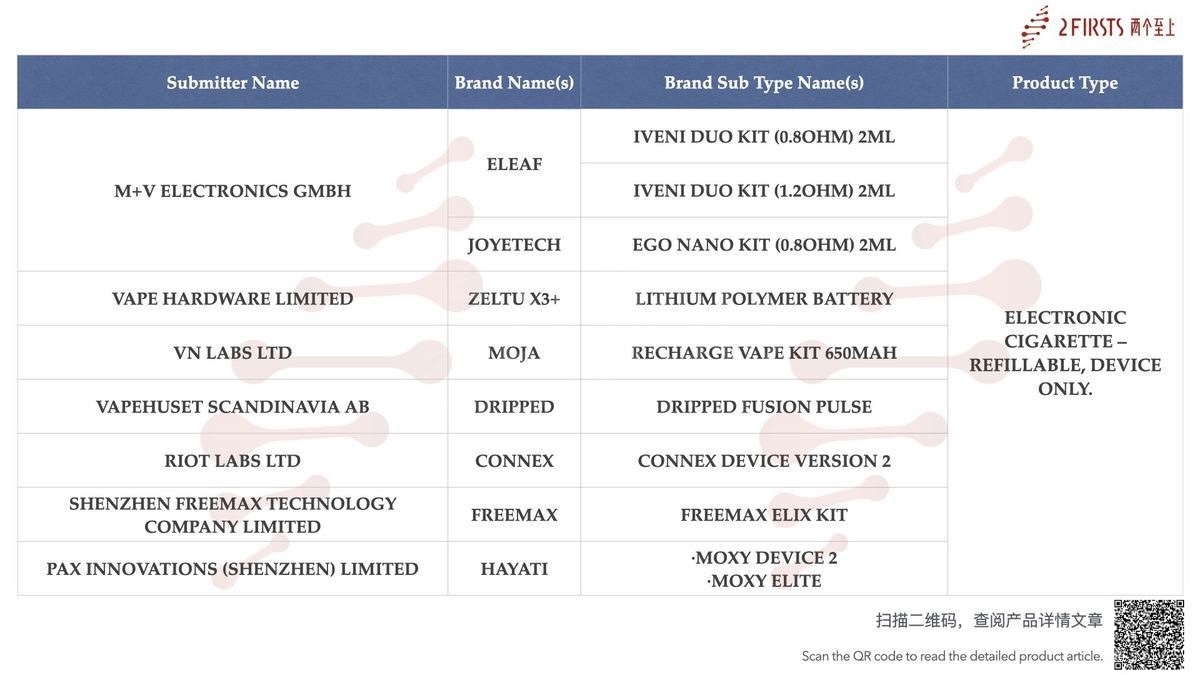
In the category “Kit – Pack containing more than one different e-cigarette device and/or more than one different refill container/cartridge”, a total of 9 SKUs were updated between August 11 and August 17, involving brands such as VAPORESSO, SMOK, and FREEMAX.
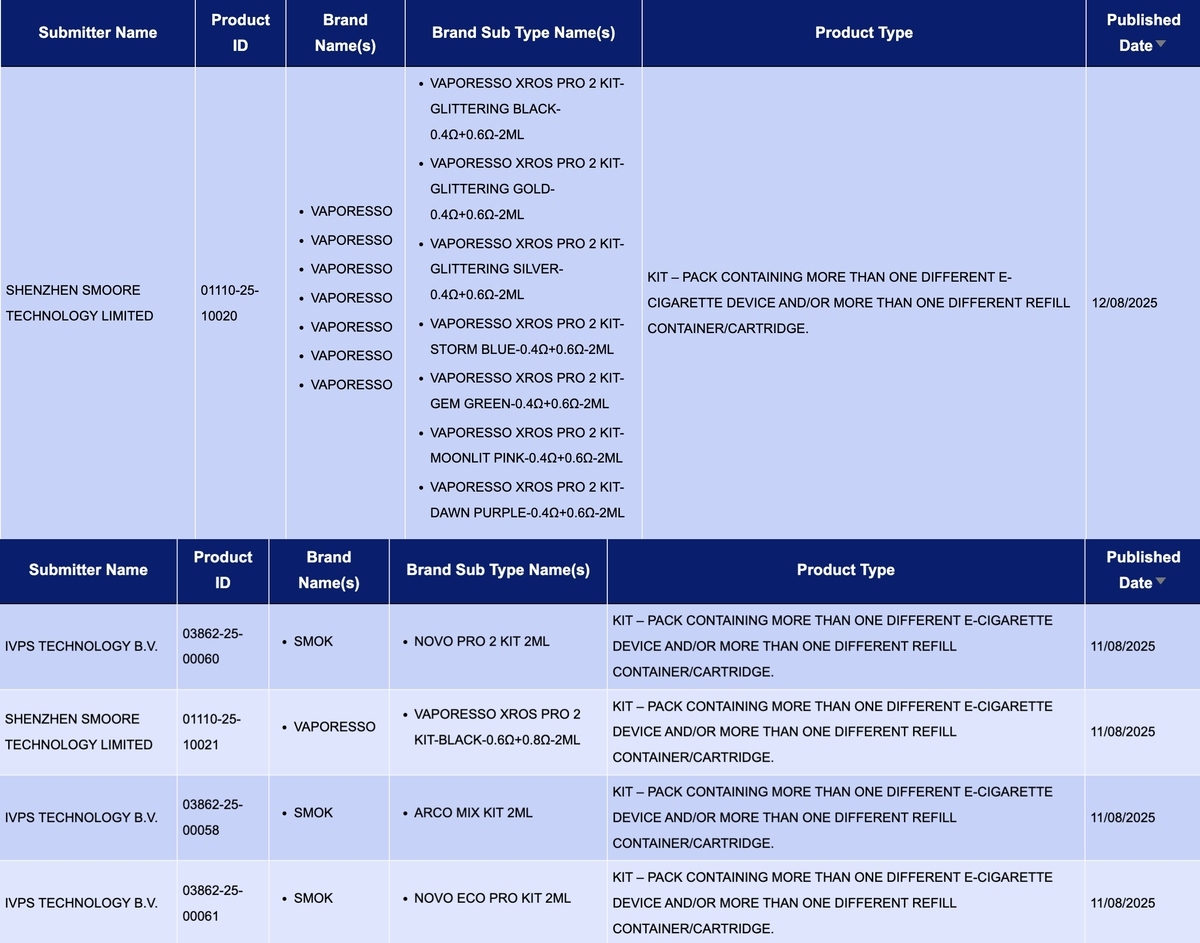
Smoore International Holdings Limited’s brand VAPORESSO has launched the VAPORESSO XROS PRO 2 KIT, available in seven colors, with specifications listed as “0.4Ω+0.6Ω-2ML.” In addition, another SKU of the same kit is available in a “0.6Ω+0.8Ω-2ML” version.
Shenzhen IVPS Technology Co., Ltd.’s brand SMOK has also published new SKUs for the ARCO S1 KIT 2ml, NOVO PRO 2 KIT 2ml, ARCO MIX KIT 2ml, and NOVO ECO PRO KIT 2ml.
In the category “Individual part of electronic cigarette capable of containing e-liquid,” a total of 65 SKUs were updated between August 11 and August 17, covering brands such as ELFBAR, HAYATI, FREEMAX, OXBAR, and CONNEX.
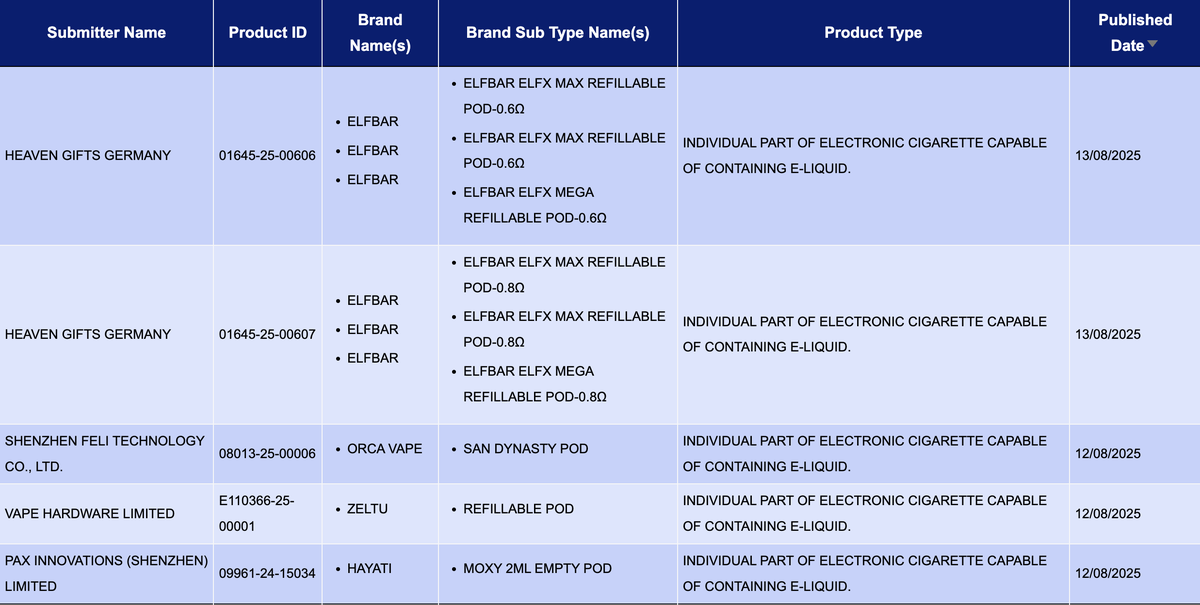
HEAVEN GIFTS GERMANY’s brand ELFBAR has published SKUs for the ELFBAR ELFX MAX and ELFBAR ELFX MEGA cartridges, with coil resistances of 0.6Ω and 0.8Ω, respectively.
Shenzhen FreeMax Technology Co., Ltd.’s brand FREEMAX has released the FREEMAX ELIX POD, featuring five SKUs with different coil resistances.
In the category “Refill container/cartridge containing e-liquid,” a total of 1,033 SKUs were updated between August 11 and August 17, covering brands such as INNOKIN, LOST MARY, PIXL, SKE, JNR, and HIGO.
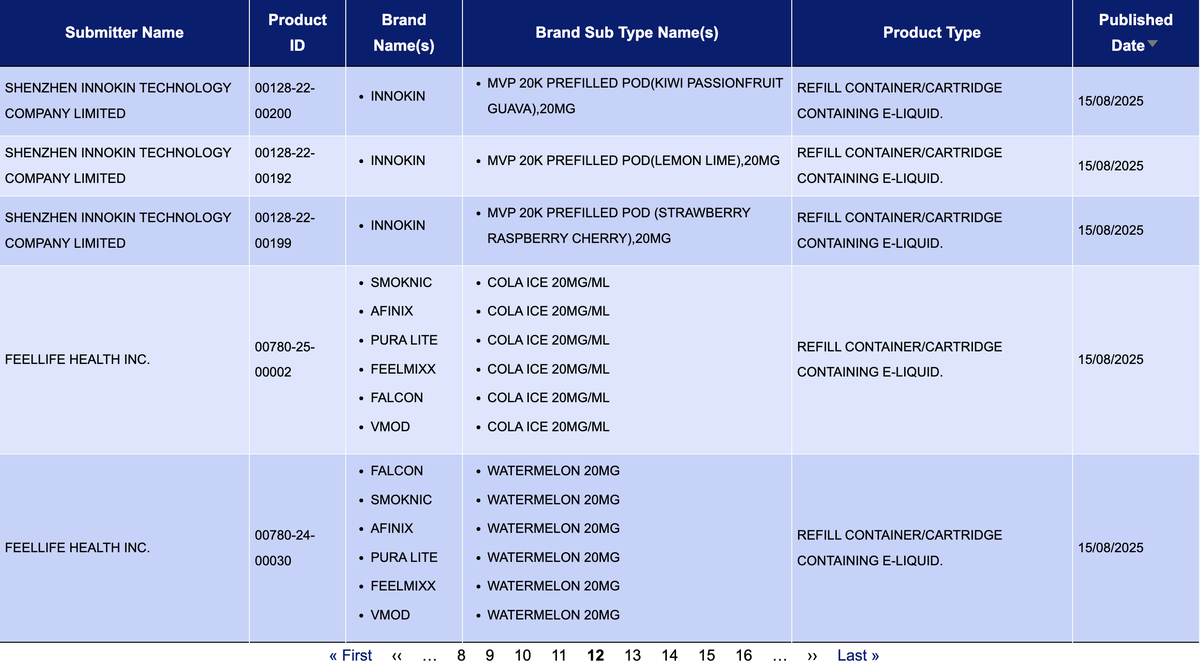
As a comparison, during the period of August 2 to 10, this category saw only 319 SKU updates, covering brands such as FEOBA, SKE, OXVA, ELFBAR, and VAPES BARS. However, between August 11 and 17, the number of updated SKUs in this category surged to more than three times higher, with all other categories also showing growth in new SKUs.
As a global leading NGP (Next-Generation Products) media and think tank, 2Firsts is dedicated to providing the latest product and technology information and insights across all NGP categories, including e-cigarettes, heated tobacco, and modern oral products. Our mission is to drive technological transformation and innovation in the global NGP industry, ultimately bringing consumers worldwide more harm-reduction products and healthier lifestyles.
With information sources spanning both China’s supply chain and global markets, 2Firsts’ product coverage has become one of the most influential platforms for new product and technology launches in the industry.
We welcome you to contact 2Firsts for:
Sharing leads on new products and technologies.
Providing commentary on products and technologies.
Seeking media coverage for your products.
Collecting information on product sales channels.
…
Contact Information:
Email: info@2firsts.com
Connect with Alan Zhao, CEO of 2Firsts, on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







