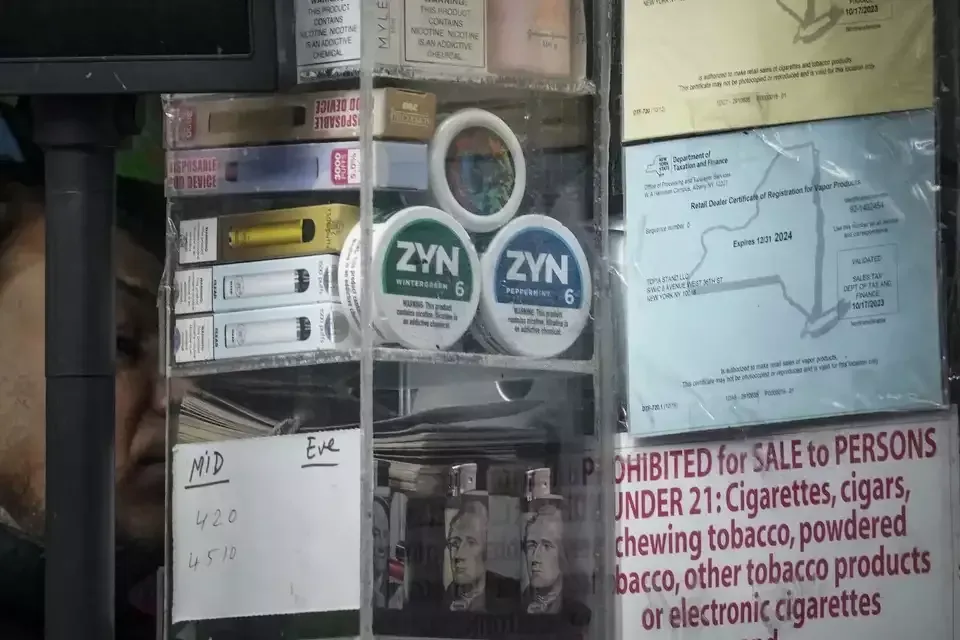
According to a report by Timesunion on February 11, the New York State Legislature is considering passing a bill to ban the sale of flavored nicotine pouches in the state. The ban is based on the belief that fruit and candy flavored products are a marketing tactic aimed at minors.
Congresswoman Linda Rosenthal has expressed concerns about the nicotine pouch brand ZYN, which offers flavors such as mint, citrus, and coffee. She pointed out that with flavored e-cigarettes becoming harder to find in New York, minors may turn to nicotine pouches.
Brian Erkkila, the Chief Scientific Officer of Swedish Match, stated that the main customer base of ZYN is not new nicotine users, but rather adults who are transitioning to safer products.
However, some experts believe that a comprehensive ban on the product may backfire. Raymond Niaura, a professor at New York University, stated that data shows the proportion of teenagers using the product is still very low, and he believes a ban is an "overreaction." Alan Mathios, a professor at Cornell University, also warned that a ban on flavored nicotine pouches could lead to the emergence of an illegal market, making it easier for teenagers to access such products.
On January 17, the U.S. FDA approved marketing authorization for 20 ZYN brand nicotine pouch products. This marks a significant milestone for the FDA in regulating novel tobacco products.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







