
Special announcement:

The information in this article comes from online users, and FIRSTS is unable to verify the authenticity of this information. We are simply providing industry news and information quickly and widely.
If you have any objections to the revelations in this article or would like to communicate with us, please feel free to contact us through the following email address: info@2firsts.com.

This article is not responsible for the accuracy of the information disclosed. Readers should maintain a rational mindset while reading and carefully evaluate the information.
In the highly competitive and saturated e-cigarette market, nicotine pouches are gaining attention as an emerging area. However, due to technology patents protection, technological barriers, and regulatory policy uncertainty, the market for nicotine pouches is flooded with counterfeit products. Addressing this issue, 2FIRSTS conducted a thorough analysis in their previous report, "Suspicions of Counterfeiting in the Nicotine Pouch Industry: Why are Fake Products So Common? How to Address the Problem of Bad Money Driving Out Good Money?" The report revealed the risks of counterfeit and compliance in the rapidly growing nicotine pouch market, highlighting the complexities of the market.
After the article was published, a senior figure in the nicotine pouch industry who is currently visiting the US market shared his observations with 2FIRSTS. According to him, counterfeit versions of the well-known brand ZYN are rampant in New York, New Jersey, Virginia, and Arizona. He described the situation as alarming, stating that during his visits he did not come across a single authentic box of ZYN in any of these four states.
Counterfeit ZYN products are now being sold in several states in the United States at twice the price of VELO.
According to reports, in the American market, in addition to the ZYN brand, there are also other nicotine pouch brands such as ROGUE, VELO, ON!, and FRE. However, ZYN is not only the most widely available brand, but also the most rampant in terms of counterfeit products.
ZYN, acquired by Philip Morris International (PMI) from Swedish Match, saw its shipment volume of nicotine pouches reach 266.7 million cans in the first half of 2024, representing a 63.5% year-on-year increase, as indicated in its performance report.
The sharp increase in sales has led to a market imbalance of supply and demand. According to industry insiders, counterfeit ZYN products are widely distributed in various retail terminals such as convenience stores, tobacco shops, e-cigarette stores, and gas stations, with prices ranging from $6.99 to $9.99, similar to authentic products. However, the nicotine pouch brand VELO, owned by British American Tobacco, is priced at only $4.99, while FRE is priced at just $2.99.
The appearance is so real that it is difficult to distinguish, and the taste is described as "sugar-like.
What is even more worrying is that these counterfeits closely resemble the authentic products in terms of appearance, including box design, color combinations, and material textures, making it almost impossible to distinguish between the two. As a result, not only do average consumers struggle to identify the authenticity of products, but even most shop owners lack a clear understanding of the authenticity of the goods they sell.
Although distinguishing between real and fake ZYN can be difficult, industry insiders have provided a viable method. They point out that the bottom of the box of ZYN contains a QR code, which consumers can scan after purchasing the genuine product to directly access the official ZYN website. If the QR code scanned is for a counterfeit product, it will first redirect to an unknown website before potentially redirecting to the ZYN official website.
In addition, authenticity can also be discerned through the use of experience. According to him, counterfeit ZYN products have significant differences in user experience compared to the genuine ones, with the fakes giving a feeling "like candy." He speculated that these imitation products may not contain any nicotine at all, or only contain a small amount of nicotine, in order to reduce production costs.
Where do counterfeit products come from?
In the article "Suspicions of Nicotine Pouch Counterfeiting: Why are Fake Products Frequently Emerging? How to Combat Bad Money Drives Out Good Money?" 2FIRSTS once held in-depth discussions with industry experts on this issue. At that time, several industry insiders pointed out that China is one of the sources of counterfeit nicotine pouches.
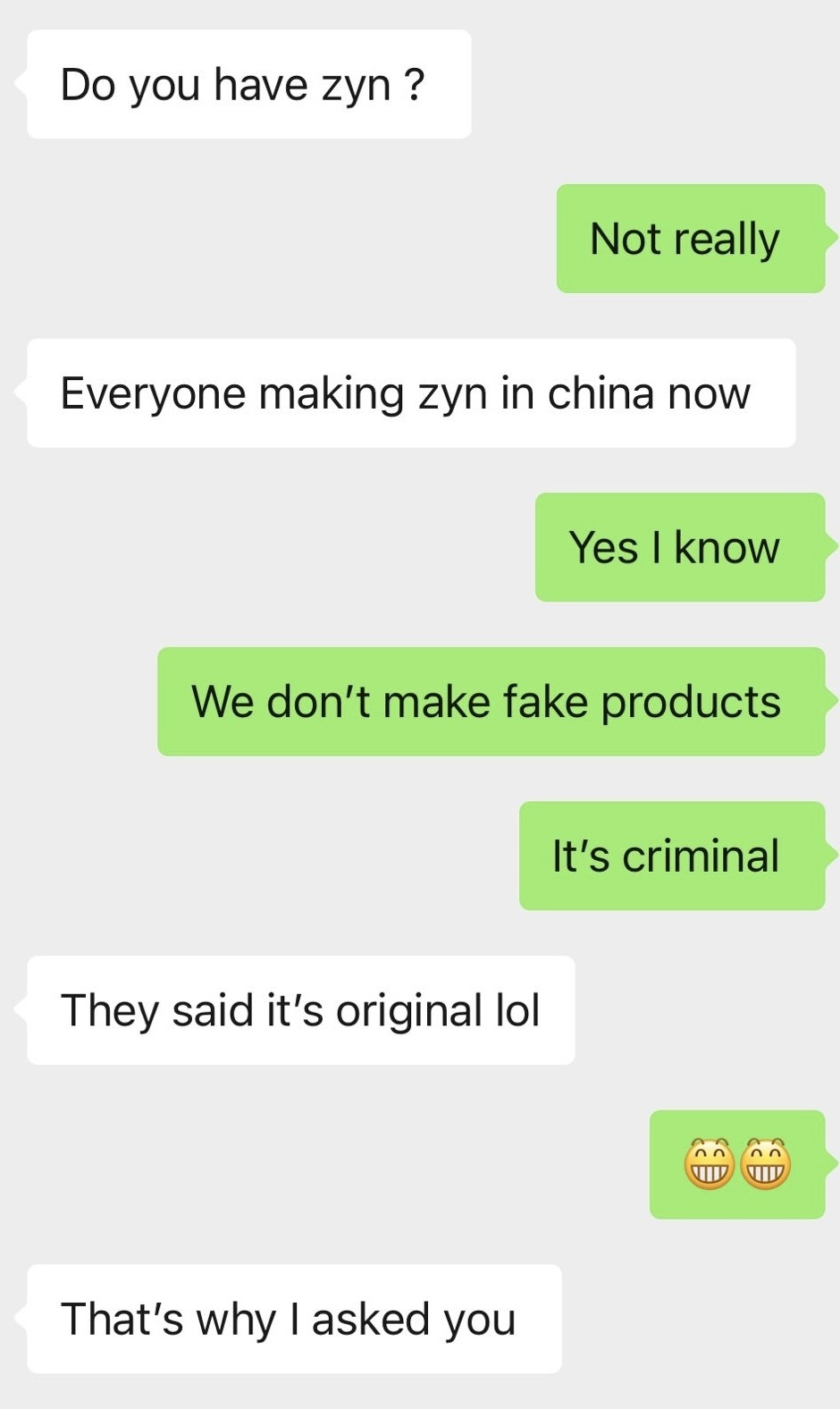
According to the investigation conducted by the aforementioned sources, the conclusion is unanimous - the majority of counterfeit ZYN products originate from manufacturers in China. Some American wholesalers even inquired about the supply chain for ZYN pouches and bluntly stated, "Everyone making zyn in china now.
However, it is understood that ZYN's official production facility is located in Sweden. Philip Morris International (PMI) announced in July of this year that a new ZYN production plant will be built in Colorado, USA. Currently, there are no officially authorized contract factories in China.
This phenomenon has already raised concerns among some industry insiders in the United States, who worry that as brands continue to expand, the issue of counterfeit goods may also arise. As mentioned by the aforementioned individuals, a client who has already obtained PMTA certification raised the question to him: if they were to manufacture products in China, would they also face the risk of being counterfeited as their business grows.
An expert in the emerging tobacco industry stated that from an industry perspective, nicotine pouches as a new form of nicotine consumption have always been controversial, especially in terms of their use by young people and health impacts. The issue of counterfeit products may exacerbate these concerns, prompting regulatory agencies and public health experts to more closely scrutinize this market.
At the same time, for legitimate manufacturers, the proliferation of counterfeit goods could damage their brand image and force them to invest more resources to protect their intellectual property and consumer rights.
For industries in China, this approach will lead to a severe lack of trust in Chinese-made nicotine pouch products overseas, causing incalculable damage to the industry's progress and development.
2FIRSTS will continue to monitor the counterfeit nicotine pouch incident and provide timely updates on the latest developments.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







