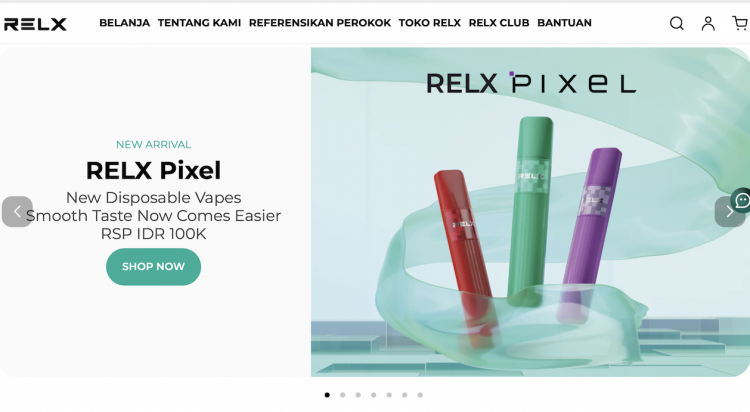
According to sources at Indonesian RELX vape shops, after releasing their new product RELX Infinity Plus on October 26th of this year, RELX has now announced another new product - one-time-use disposable product RELX Pixel. On November 1st, RELX Pixel was officially launched for sale both online and in physical stores in the Indonesian market.
A new product priced at 100,000 Indonesian rupiahs (approximately 46 yuan in Chinese currency) is now available in four flavors: Ludou Lce, Lemon Zest, Watermelon Ice, and Tangy Crape. The product is manufactured by SOMO and features FEELM ceramic cores.
The PR head of RELX Southeast Asia told 2FIRSTS that their product is globally launched in Indonesia, and will be introduced in other countries in the future, but the timeline is unspecified. The price of 100,000 Indonesian rupiahs is carefully chosen to be competitive in the local market.
On November 1st, the 2FIRSTS frontline staff visited several RELX Yueke offline flagship stores and found that the product had great first-day sales. One flagship store sold out of its inventory of one thousand units by the morning.
Product details. Photo source: Luofeng from 2FIRSTS. Product details. Photo source: Luofeng from 2FIRSTS. Product details. Photo source: Luofeng from 2FIRSTS. Product details. Photo source: Luofeng from 2FIRSTS.
RELX Pixel comes in 4 different flavors, one of which is Tangy Crape4. Image source: Official RELX website.
RELX YS has four different flavors of their Pixel product, with Lemon Zest being one of them. Image source: RELX YS official website.
The RELX Pixel has four flavors, including Watermelon Ice and Ludou Lce, according to sources on the company's official website.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.








