
Recently, Smoore International Holdings Limited (06969.HK, "Smoore International") released its "2024 Environmental, Social and Governance Report". The report contains information on the company's annual progress in product and technology innovation. Data from the report shows that Smoore invested approximately 1.57 billion RMB in research and development in 2024, with 1609 R&D personnel. The company has established a research and development system including research institutes, technology centers, product development teams, and technology industrialization centers.

The report by Firsts found new developments in the field of electronic atomization technology in the year 2024.

FEELM technology platform: POWER ALPHA 2.0 version supports over 15,000 puffs, with a battery energy density reportedly increased by 40% and a 70% reduction in self-discharge rate.
The FEELM Turbo ceramic core solution is said to increase flavor intensity by 200%, vapor production by 80%, and puff count by over 30%.
FEELM Pro brings two different series of high puff count solutions: the TPD compliant 5000 puff solution TANKER and the TPD compliant 3200 puff four-in-one solution QUAD SHOT Pro.
VAPORESSO, a self-owned brand, has launched the XROS, LUXE, ECO, and ARMOUR series products. The company has also introduced the VIBE product platform, with a focus on providing a similar taste and ease of use as disposable products, along with stylish design.
The taste emphasizes delicacy, richness, and consistency; ease of use emphasizes large capacity, adjustability, easy pod replacement, and strong battery life; the design style aims for a young and trendy look, incorporating integrated design and screen display technology.
The report notes that VAPORESSO products have won several awards, including the MUSE Design Award, London Design Award, IDA International Design Award, French Bilateral Design Award, German Design Award, and International CMF Design Award.

In other areas of atomization applications, the report disclosed the following information:
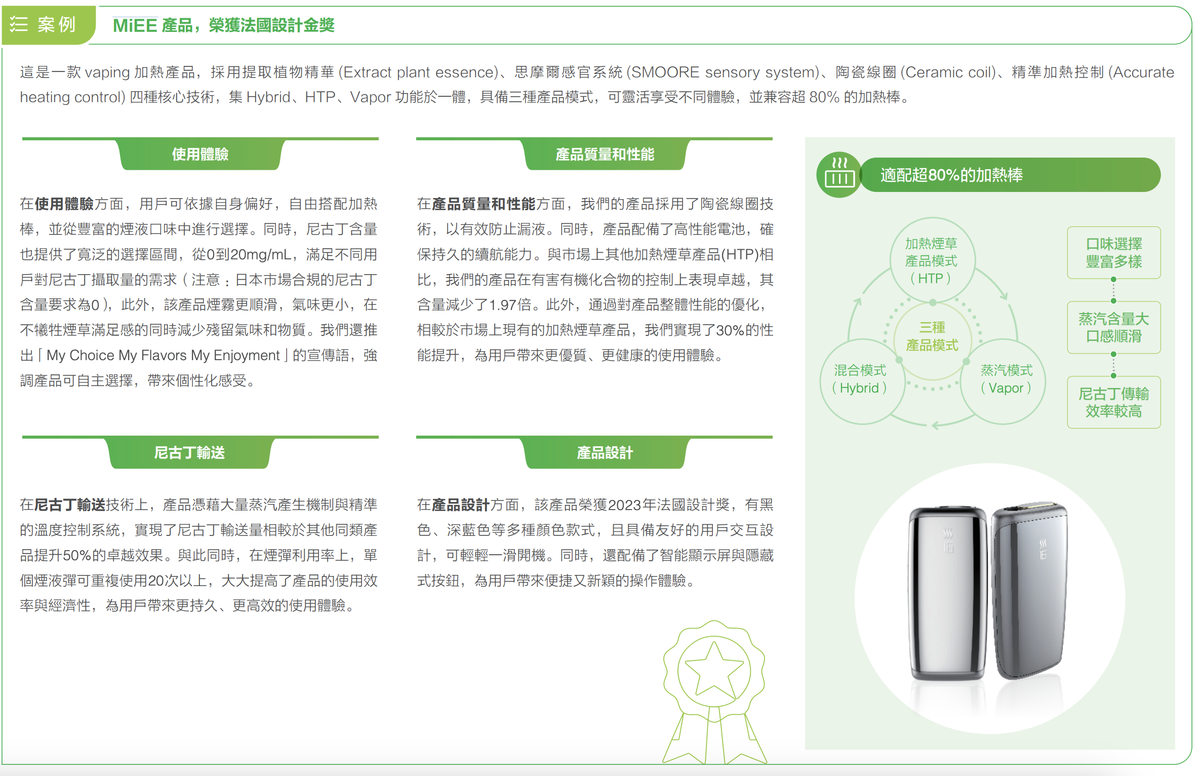
HNB (Heated Tobacco Product)/Hybrid/e-cigarette product: The MiEE product combines heating tobacco and vaporization functions, using plant extract technology that is compatible with over 80% of heating sticks. The report states that this product reduces harmful organic compounds by 1.97 times compared to other HTP products, improves performance by 30%, and increases nicotine delivery by 50%.
Moyal launched its MOYAL Rain brand in 2024, featuring TPS transdermal technology to aerosolize high-viscosity essence for skin penetration. The product line includes both consumer and professional-grade devices (2B+2C).
In the field of aerosolized medicine, its subsidiary Transpire Bio completed the introduction of inhalation devices for asthma and COPD, with key indicators meeting European and American regulatory requirements. Collaborations with international pharmaceutical companies have been established to advance the development of integrated medications with inhalation delivery devices (2B+2C).
The report also highlights an iF Design Award-winning cartridge-based product, utilizing 100% tobacco extract and incorporating a rotating pop-up hygienic mouthpiece.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







