The Wall Street Journal reported on June 22nd that insider sources revealed the UUL Labs brand of electronic cigarettes may be required to withdraw from the US market by the Food and Drug Administration (FDA). The FDA could potentially announce their decision as early as Wednesday.
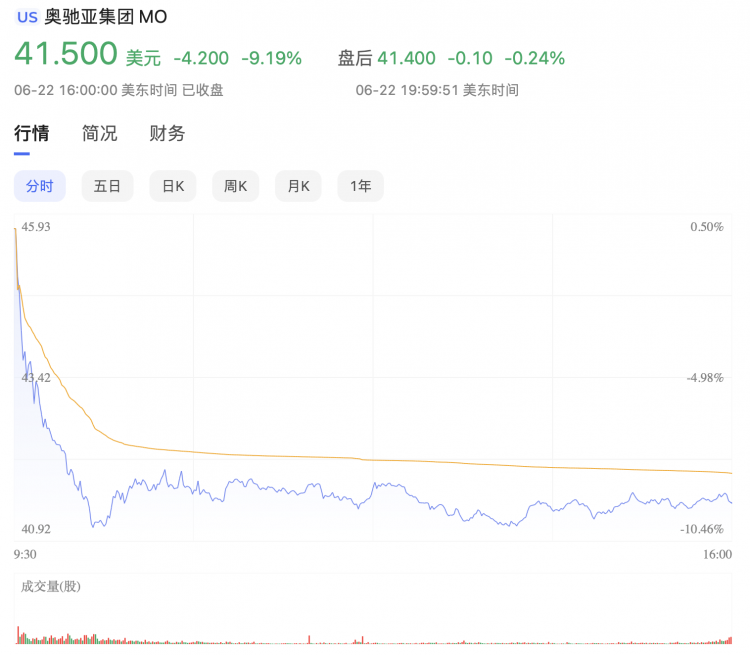
As a result of this news, tobacco giant Altria Group, which owns a 35% stake in JUUL, experienced a sharp decline in stock price on Wednesday, giving back all gains made throughout the year. At the close of trading on Wednesday, Altria had fallen 9.19% to $41.50 per share.
In July 2020, JUUL Labs submitted a PMTA in order to obtain authorization for the continued sale of its tobacco and mint-flavored e-cigarettes on the US market. However, after a two-year review of the data submitted by JUUL, the US FDA is reportedly set to reject the company's application, according to a report by the Wall Street Journal.
According to Stifel analyst Christopher Growe, the ban on flavored e-cigarettes was unexpected because Altria had already been successful in decreasing underage use by reducing the attractiveness of their products to young people. Jefferies analyst Owen Bennett added that the PMTA review of Vuse Alto could also be affected by recent surveys showing a higher rate of underage use compared to JUUL.
Four years ago, JUUL came into the sights of the US FDA. In 2018, following Altria's acquisition, JUUL management decided to issue a year-end bonus worth $2 billion, averaging around $1.3 million per person for its 1,500 employees. This was JUUL's shining moment, as the company's e-cigarette products had a 70% market share in the US and were especially popular among American high school students. However, the company's fruit-flavored e-cigarettes and trendy marketing techniques were criticized for promoting a wave of underage drug use. Since then, the company has been working to regain trust from regulators and the public, limiting its marketing activities and ceasing the sale of sweet and fruit-flavored e-cigarettes in 2019. In recent years, JUUL's sales have seen a decline.
2FIRSTS, a tobacco lawyer, stated that JUUL may consider taking legal action against the FDA.
Consulting with senior tobacco industry lawyer Tang Shunliang regarding the recent news about 2FIRSTS, Tang stated, "There is currently no official FDA announcement about JUUL's market withdrawal, so we need to wait for further confirmation. However, the FDA has previously mentioned in letters and other documents to electronic cigarette companies that the purpose of e-cigarette products is to help consumers reduce their dependence on tobacco and have alternative smoking options. In reality, many young people (adolescents) who do not smoke have become addicted to nicotine in e-cigarettes, so even if the rumors are true, the FDA's actions are supported by a large amount of data. Specifics still await the FDA's official announcement to determine if all or only one of JUUL's products will be withdrawn. From JUUL's standpoint, in accordance with relevant US laws and the FDA's defense mechanisms, if there is indeed a dispute and JUUL decides to refuse, JUUL will still take legal action to fight for its case.
According to a report in the Wall Street Journal citing sources, the FDA is set to officially issue a refusal letter for JUUL's PMTA in the coming days. As of now, JUUL has not responded to these rumors and 2FIRSTS will continue to closely monitor developments regarding this matter.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.







