Special statement:
1. This article is intended for internal industry communication only and does not make any recommendations for specific brands or products.
2. The images displayed in this article are solely used for describing facts and are not intended as advertisements for any products.
3. Minors are prohibited from accessing this article.
Product Highlights:
1. Significant increase in the number of puffs: The number of puffs has been raised from 36,000 in previous products of the same series to 50,000, enhancing the durability and usage time of the product.
2. Upgrade in appearance: The product's appearance has been improved from single-color spray painting to gradient technology.
3. Technological improvements: With no change in product size, weight, atomizer core, or battery capacity, there has been a significant increase in the number of puffs. 2FIRSTS thus speculates that there may have been optimizations in the product's power.
4. Market competitiveness: The product has been launched on various distributor websites in the United States, with a price of around $16.99.
5. Brand background: Launched by Shenzhen AileyMi Technology Co., Ltd., established in 2017, which is the parent company of electronic vaporizer brands OXVA and OXBAR.
2Firsts has observed that e-cigarette brand OXBAR recently showcased a new e-cigarette model—OXBAR Astro Maze 50K on its official website, which has simultaneously been listed on multiple e-cigarette distributor websites in the United States, priced at around $16.99.
In December 2024, the OXBAR brand launched the OXBAR GTURBO e-cigarette in the Canadian market. From the appearance of the product, the OXBAR GTURBO seems to be a different version of the same series as the new OXBAR Astro Maze 50K.
Two FIRSTS compared the product parameters of the OXBAR GTURBO and the new OXBAR Astro Maze 50K, as shown in the table below:
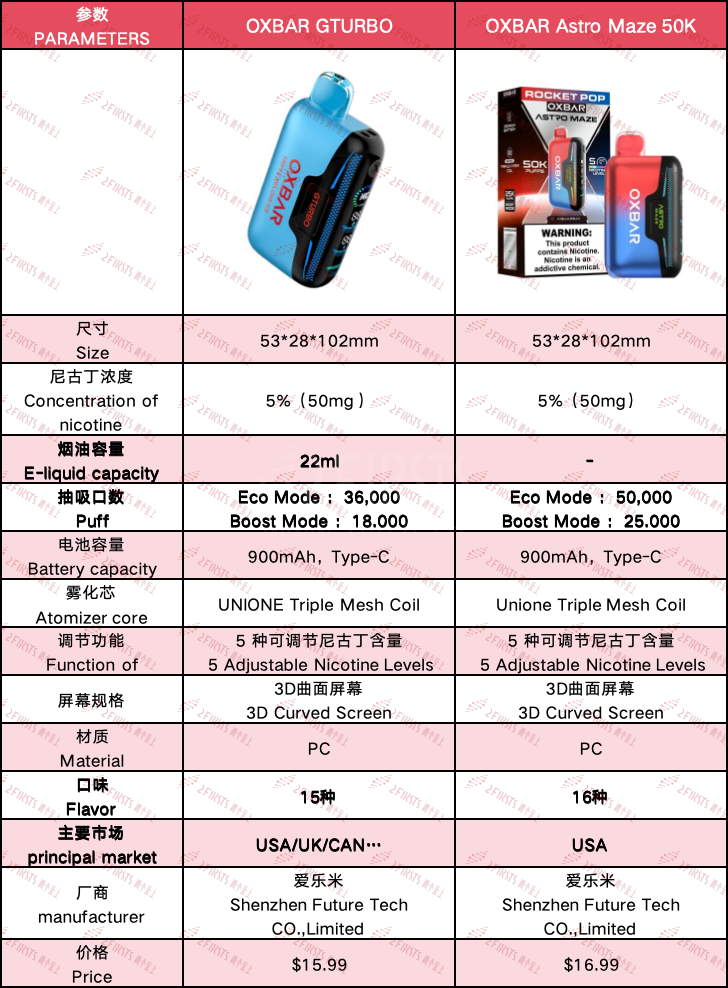
In comparison to the OXBAR GTURBO e-cigarette, the new OXBAR Astro Maze 50K has been upgraded in terms of the number of suction mouths, product flavors, and exterior paint.
The product now features 50,000 suction mouths, making it more competitive in the e-cigarette market in the United States.
In addition, the number of product flavors has increased from 15 to 16, including flavors such as watermelon ice and cherry grape. The exterior appearance of the product has also been upgraded from the previous single-color spray paint to a higher-cost gradient process.


It is worth noting that the previous OXBAR GTURBO had a 22ml e-liquid capacity, corresponding to 36,000 puffs; while the new OXBAR Astro Maze 50K has the same product dimensions, product weight, atomizer core, and battery capacity, but the number of puffs has increased to 50,000.
According to analysts at 2FIRSTS product researchers, based on the public information of the product, it is speculated that the OXBAR Astro Maze 50K e-cigarette may have undergone adjustments in product power.

In terms of market expansion, OXBAR GTURBO was first launched in the Canadian market in December last year, followed by its availability on e-cigarette distributor websites in the UK, US, and other countries.
OXBAR Astro Maze 50K has also recently started appearing on the OXBAR brand website and several e-cigarette distributor websites in the US, following a different market strategy.
In addition, the OXBAR Astro Maze 50K is a product launched by the e-cigarette brand OXBAR, which is owned by Shenzhen Ailemi Technology Co., Ltd.
Ailemi was established in 2017 and is based in Bao'an District, Shenzhen. It is the parent company of the electronic vaporizer brands OXVA and OXBAR.
The 2FIRSTS product column is dedicated to providing readers with the latest information on new products in the tobacco industry. In order to gather and share firsthand information from the industry, 2FIRSTS welcomes readers to submit articles and share the latest products in the e-cigarette field.
If you have any unique insights or information, please feel free to contact us at info@2firsts.com, or reach out to 2Firsts CEO Alan Zhao on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com