
Disclaimer
- This article is intended solely for internal industry communication and does not constitute an endorsement of any brand or product.
- The images presented in this article are for factual illustration purposes only and should not be interpreted as advertisements for any product.
- Access to this article is prohibited for minors.
2Firsts has observed that several vape brands have recently launched their own disposable shisha-style vape products. Vape brand HAYATI also introduced the Hayati Shisha 15000 to the UK market, now available on local vape distributor websites.
Key Features of the Hayati Shisha 15000:
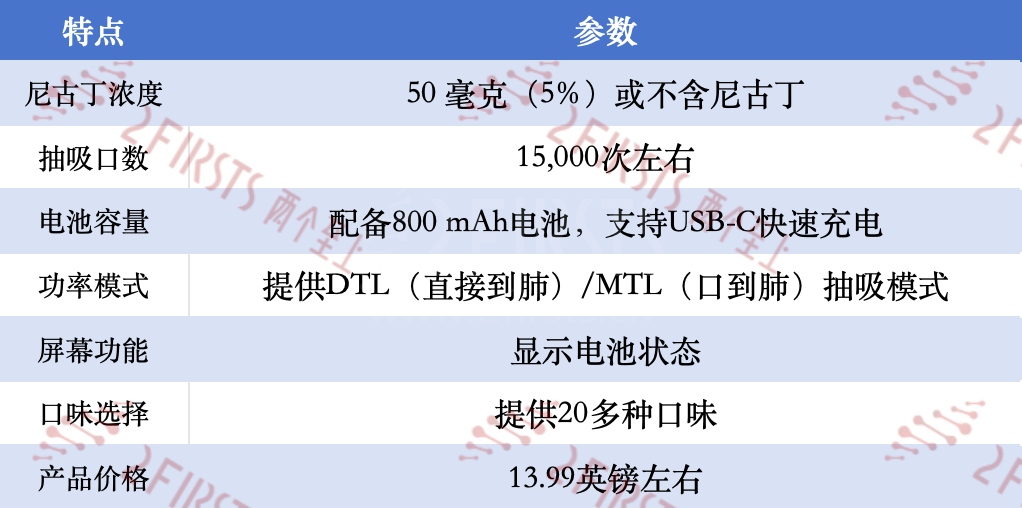
Hayati Shisha 15000 stands out with its choice of two nicotine levels: 50 mg (5%) or nicotine-free, and it supports both MTL (mouth-to-lung) and DTL (direct-to-lung) vaping modes.

In terms of design, like recent disposable shisha-style vape products from brands such as HQD, OXBAR, and Kangvape, the Hayati Shisha 15000 adopts a cylindrical shape, aligning with current market trends for disposable shisha vapes.
The Hayati Shisha 15000 has already appeared on UK vape retailer websites like vapesourcing.uk, ideavape, and ninja-vapes with a status marked as "COMING SOON." However, it has not yet been featured on the official HAYATI brand website.
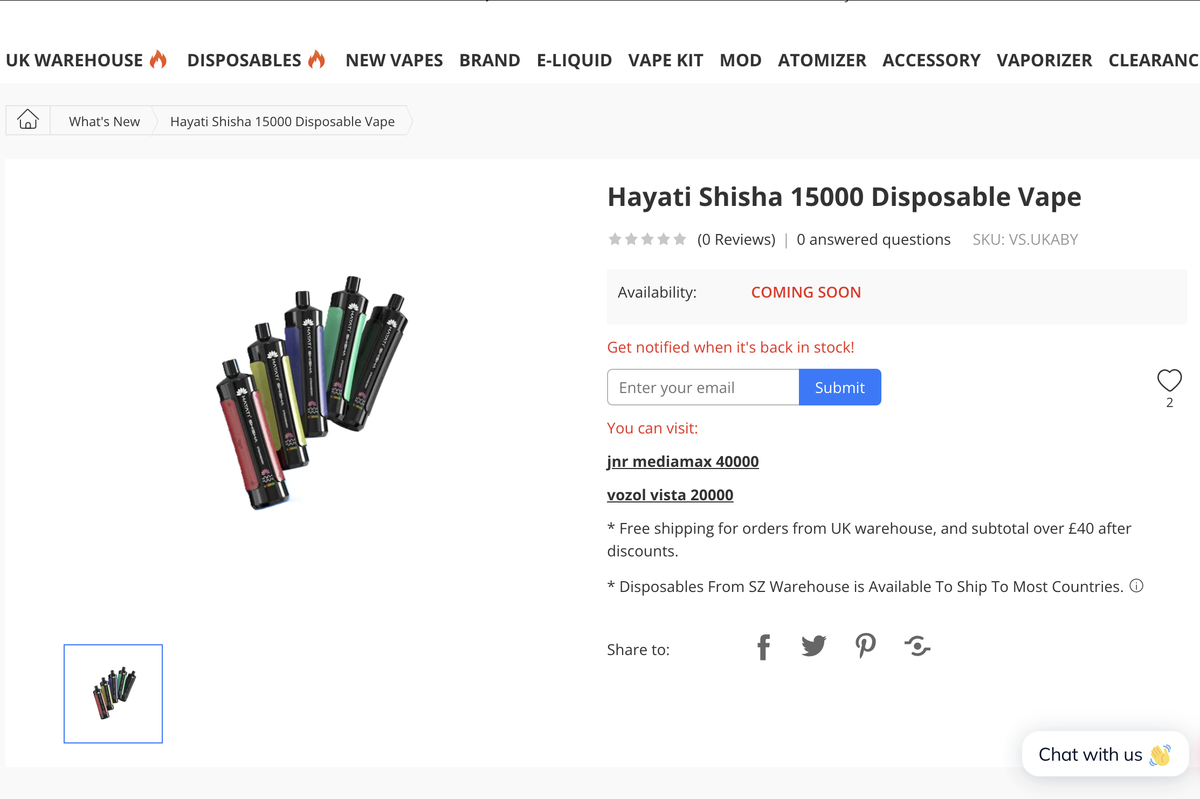
Notably, the Hayati Shisha 15000 is not listed in the UK Medicines and Healthcare Products Regulatory Agency (MHRA) vaping products database.
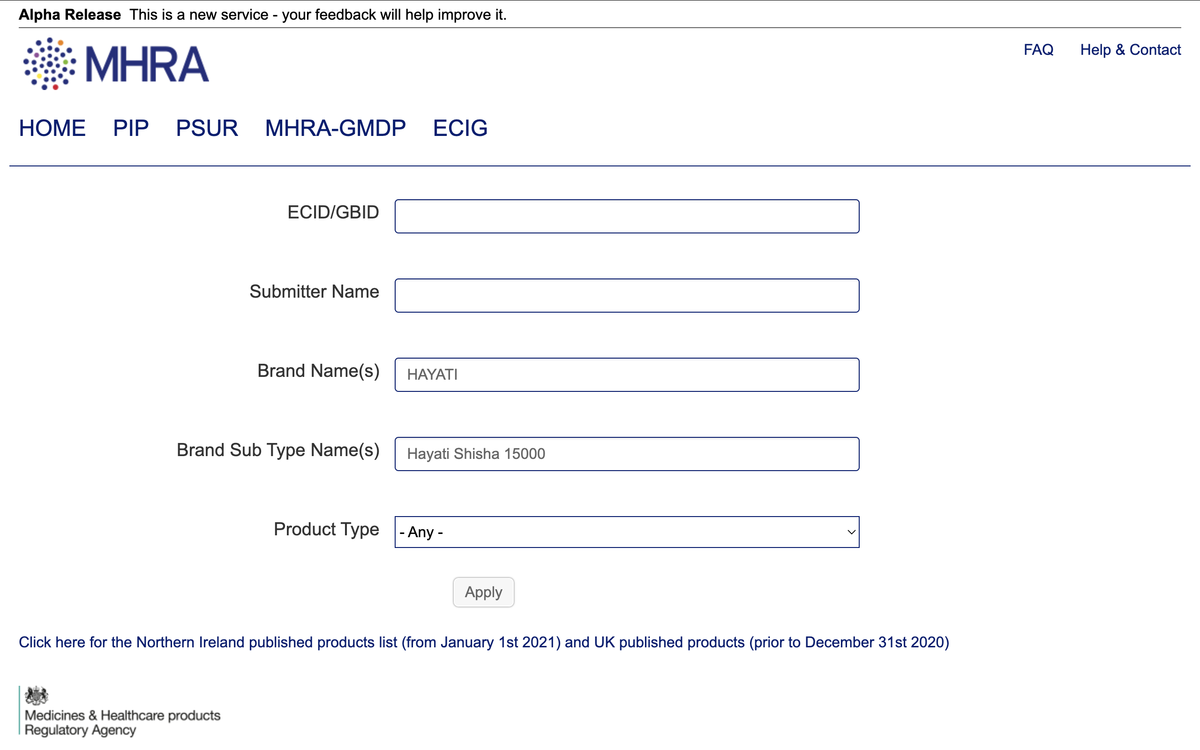
According to the HAYATI official website, the brand belongs to PAX Innovations (Shenzhen) Limited, which submits all product registration applications to the MHRA. The website also mentions "PAX Manufacturing" and provides a relevant link.

PAX's official website states that the company was established in Hong Kong in 2021. Its founder, Mr. He, is a veteran expert in the vape industry with a long history of research and innovation in e-cigarette products.
2Firsts will continue to track trends and developments in the disposable shisha-style vape product market, ensuring the public receives accurate and authoritative industry updates.
2Firsts' product column remains committed to delivering readers the latest product updates in the new tobacco field. We invite readers to submit insights and news on e-cigarette developments.
For unique perspectives or information, please get in touch with us at info@2firsts.com.







