
As smokers increasingly seek safer alternatives, tobacco-free nicotine pouches (TFNPs) are gaining attention as a promising tool for tobacco harm reduction (THR). In an interview with 2Firsts, Dr. Erika Grandolfo, Scientific Affairs Manager at Imperial Brands, highlighted the company's research into TFNPs such as zoneX, which contain significantly fewer harmful chemicals than cigarettes and offer a slower release of nicotine, reducing the risk of addiction while still satisfying adult smokers.
Dr. Grandolfo also discussed Imperial's broader focus on next generation products (NGPs), including heated tobacco and vapor products. She emphasized the importance of evidence-based regulation that supports adult smokers' access to these safer options, while preventing youth uptake and misuse.

- "TFNPs like zoneX contain substantially lower levels of harmful chemicals compared to cigarette smoke, and even conventional tobacco-containing snus.”
- “You can create the best reduced risk product on the market in terms of its scientific profile, but if the consumer doesn't choose it – then it won’t make any contribution to tobacco harm reduction."
- "Policy should always be driven by a solid base of rigorous scientific evidence – not human emotion."
- "Regulators and policy makers should listen more to consumers to really understand their needs, and how to better support and protect them – rather than simply telling them what to do."
Tobacco-free nicotine pouches: A safer alternative for adult smokers gains momentum
At the InterTabac event in Dortmund this October, the focus was on emerging products designed to reduce the risks associated with traditional tobacco use. Dr. Grandolfo, Scientific Affairs Manager at Imperial Brands, emphasized the rising significance of tobacco-free nicotine pouches as a key innovation in tobacco harm reduction.
"Imperial is playing our part in contributing to the growing scientific weight-of-evidence demonstrating their tobacco harm reduction (THR) potential," Dr. Grandolfo explained in an interview with 2Firsts. "Our products, such as zoneX, contain far fewer – and lower levels of – harmful chemicals compared to both cigarette smoke and traditional tobacco snus."

Research conducted by Imperial Brands highlights that the biological responses triggered by TFNPs in laboratory studies are significantly less severe than those caused by cigarette smoke. This suggests that using TFNPs could result in a lower overall health risk. While TFNPs still efficiently deliver nicotine into the bloodstream through the lining of the mouth, the process is slower and less intense compared to smoking.
"This suggests it has a lower abuse liability compared to cigarettes – although this shouldn’t be interpreted as evidence that zoneX isn’t addictive," Dr. Grandolfo said.
From a practical perspective, TFNPs offer a satisfying alternative for adult smokers who are looking to reduce or quit smoking. "It has an encouraging short-term safety and tolerability profile, with no serious product-related adverse events observed under the conditions of controlled clinical trials," Dr. Grandolfo said.
Importantly, the appeal of TFNPs is largely restricted to adult smokers, with minimal interest from non-smokers, which addresses concerns about the potential for unintended use.
Imperial Brands' science-backed harm reduction strategy

Dr. Grandolfo detailed Imperial Brands' extensive research into next generation products, a category that includes heated tobacco products (HTPs), electronic vapor products (EVPs), and oral nicotine delivery products like TFNPs. These products are part of the company's commitment to providing safer alternatives to traditional tobacco, helping smokers reduce health risks.
"At Imperial Brands we’re committed to starting with the consumer; they are at the forefront of everything we do. After all, you can create the best reduced risk product on the market in terms of its scientific profile, but if the consumer doesn't choose it – then it won't make any contribution to tobacco harm reduction," Dr. Grandolfo said.
To address this, Imperial Brands has developed a diverse portfolio of NGPs designed to replicate the smoking experience without the harmful effects of tobacco combustion. These products undergo rigorous safety and quality control processes to ensure they meet high standards.
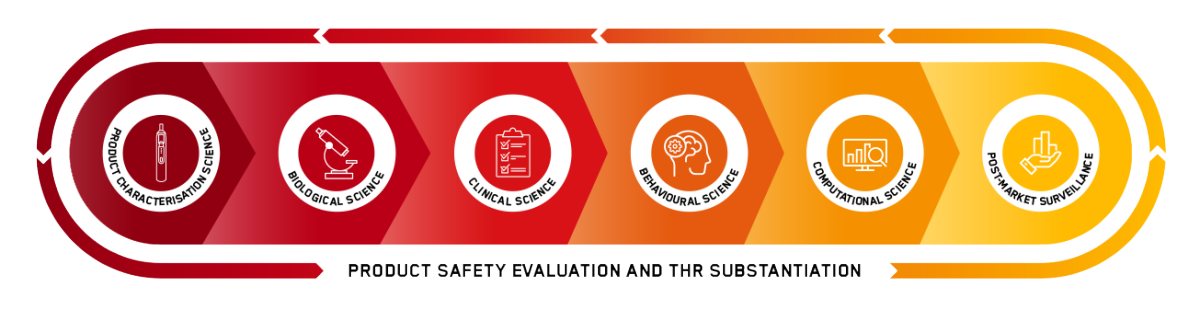
Central to this effort is the company’s Scientific Assessment Framework (SAF), a multi-disciplinary evaluation system. The SAF assesses NGPs across a range of critical areas, including product characterization, biological science, clinical trials, behavioral science, computational science, and post-market surveillance. The findings from each of these studies create a robust body of evidence supporting the harm reduction potential of NGPs.
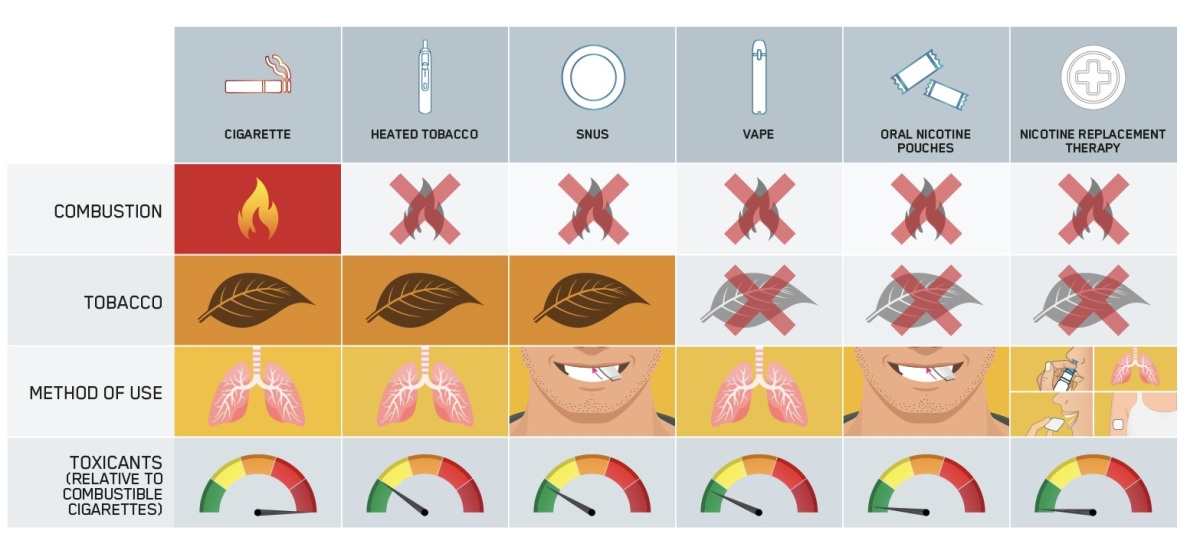
Dr. Grandolfo also introduced the company’s Relative Risk Scale (RRS), a tool designed to illustrate the risks of various nicotine products in comparison to traditional cigarettes. “The RSS demonstrates the distinction between high-risk cigarettes and other, potentially significantly less harmful nicotine-containing products that don’t involve tobacco combustion,” she said.
Why risk-proportionate regulation is essential for tobacco harm reduction
According to Dr. Grandolfo, tobacco harm reduction is not just theoretical but a proven public health strategy, with real-world examples like Sweden’s success in reducing smoking rates and smoking-related diseases through the introduction of snus. Despite this achievement, she pointed out that Sweden's experience has not been adequately recognized by regulators, public health bodies, or the media.
"Policy should always be driven by a solid base of rigorous scientific evidence – not human emotion," Dr. Grandolfo said.
She explained that risk-proportionate regulation is critical for ensuring that reduced-risk products, such as TFNPs, are treated differently from traditional cigarettes. This includes having advertising regulations and tax policies that reflect the lower health risks of non-combustible products.
However, she stressed that marketing must responsibly target adult smokers while strictly avoiding youth engagement.
Dr. Grandolfo also said the importance of product quality and responsible marketing across the industry. She called for stronger collective efforts to prevent substandard devices, adulterated e-liquids, and unethical marketing practices, which could jeopardize the overall goal of reducing tobacco-related harm.
"Regulators and policy makers should listen more to consumers to really understand their needs, and how to better support and protect them – rather than simply telling them what to do," she said.
NGP harm reduction research lacks long-term data
Despite significant progress in research on NGPs, Dr. Grandolfo acknowledged that significant challenges remain. Since 2010, more than 10,000 scientific studies on vaping have been published, with the majority confirming the harm reduction potential of these products compared to traditional cigarettes.
However, "there will always be some studies focus only on absolute risk to non-smokers, rather than relative risk compared to continuing to smoke cigarettes," she said.
One of the biggest obstacles is the lack of long-term epidemiological data. While this remains a challenge, Dr. Grandolfo noted existing real-world evidence from countries such as Sweden, Japan and New Zealand, where products such as snus and heated tobacco have successfully reduced smoking rates.
Dr. Grandolfo also emphasized Imperial Brands' commitment to ongoing research and innovation. She highlighted a recent behavioral study conducted in the Czech Republic which demonstrated the potential of Imperial's PULZE & iD heated tobacco system to help adult smokers significantly reduce – or even entirely switch away from – smoking.
Proposals for strengthening China's NGP supply chain

China plays a pivotal role in the global supply chain for NGPs, and Imperial Brands is investing significantly to meet the evolving demands of consumers. Dr. Grandolfo highlighted the importance of China in this ecosystem, particularly with the company's recent opening of a consumer innovation facility in Shenzhen. This facility enables Imperial Brands to work closely with local partners and accelerate the development of new products.
However, Dr. Grandolfo identified key areas for improvement within the Chinese NGP supply chain. She said: "Regarding potential improvements within China’s NGP supply chain, we humbly suggest investment in the more widespread use of certified (ISO17025) laboratories for independent testing. This would undoubtedly contribute to more reliable test results for submission to regulatory authorities.”
Dr. Grandolfo also advocated the wider adoption of certified design and manufacturing processes: "From a design perspective, this would have a hugely positive impact on product safety via risk assessments and validated mitigations. From a manufacturing point of view, it would result in better-qualified processes, testing, inspections, and training."
Bridging gaps in scientific communication for THR
In terms of disseminating her research, Dr. Grandolfo stressed that accurate and consistent communication of scientific research is critical to advancing tobacco harm reduction.
She acknowledged that the frequent lack of alignment between industry, regulators, and public health agencies adds to the challenge of widely communicating their encouraging NGP data. To ensure transparency, she said: "We continue to invite critique of our science through the process of peer-review in scientific journals, the regular presentation of our research at international conferences like InterTabac, and by transparently publishing all our research on our dedicated science website www.imperialbrandsscience.com"







