
According to a report from Audacy on October 17, New York City Mayor Eric Adams announced on that day that over 1200 pounds of illegal e-cigarette products have been seized and are currently being prepared for transport out of New York City for destruction. The products to be destroyed include several well-known e-cigarette brands such as YOOZ and AIR BAR.

The seized products by the task force contain batteries and other hazardous materials that require safe handling. The products will be sent to ENP Environmental, a long-term DNA evidence destruction supplier for the New York Police Department (NYPD), located in Ohio, for processing.
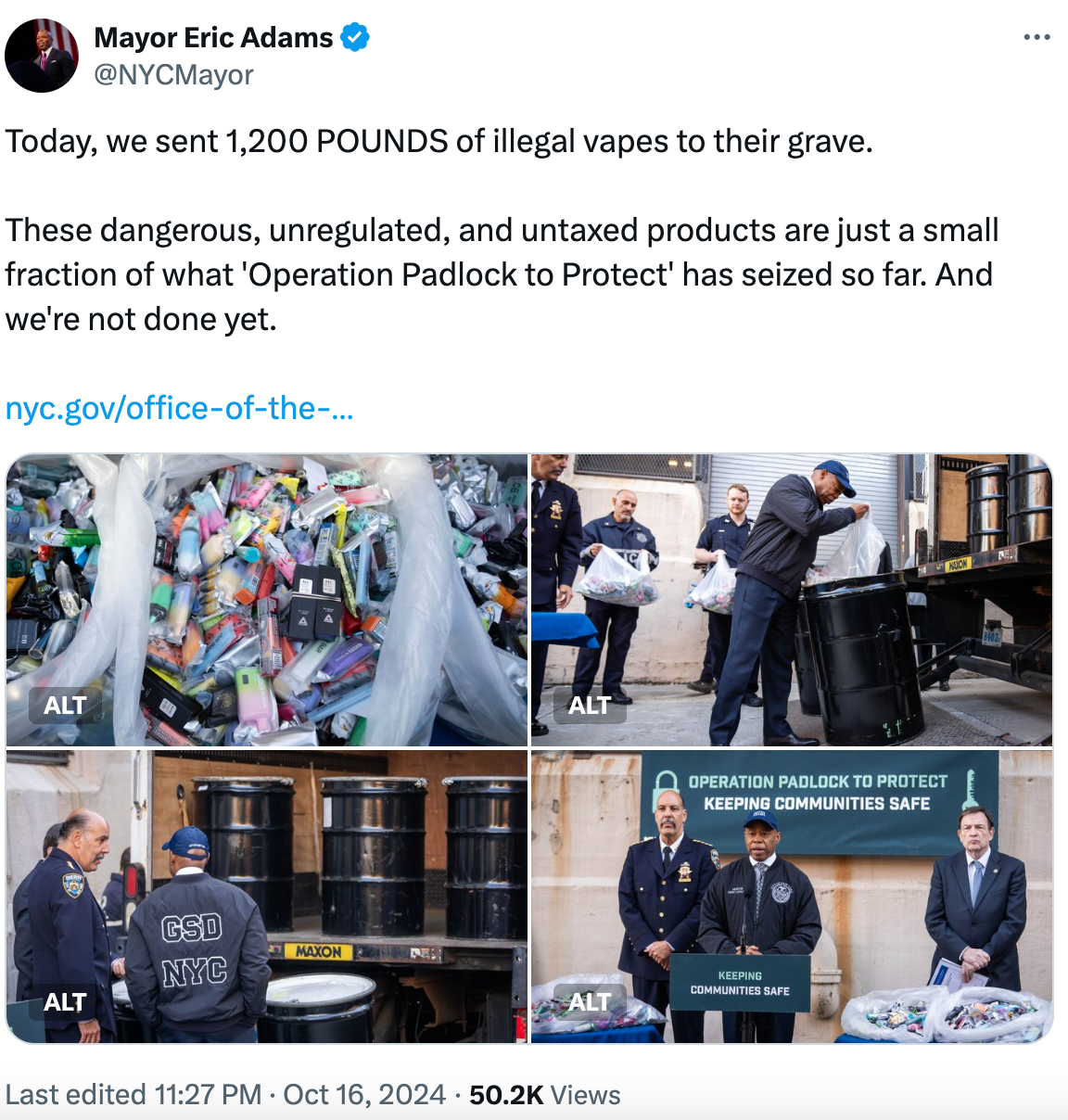
As of now, the City of New York has seized illegal goods worth over 80 million US dollars (61.44 million pounds) in this operation, which are taking up too much space in the NYPD evidence warehouse. In August of this year, the task force destroyed over four tons of illegal marijuana products, as part of the standard procedure for disposing of illegal items by the NYPD.
On October 16th, Mayor Adams announced on his social media platform X that a total of 1200 pounds of illegal e-cigarettes have been seized. He stated, "These dangerous, unregulated, and untaxed products are just a small portion of what has been confiscated as part of the 'Operation Lockdown' initiative." He added that the crackdown on these illicit products is still ongoing.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








