The Shenzhen Tobacco Monopoly Bureau has issued a notice regarding the release of the quality supervision and spot check plan for e-cigarette products sold in Shenzhen in the second half of 2024.

According to the relevant provisions of the "Implementation Rules for Quality Supervision Spot Checks of e-cigarette Products" (National Tobacco Bureau [2022] No. 59), the Shenzhen Tobacco Monopoly Bureau has organized the formulation of the "Supervision and Spot Check Program for the Quality of e-cigarette Products Sold in Shenzhen in the Second Half of 2024," which is now being announced.
The Shenzhen Tobacco Monopoly Bureau
September 29, 2024
The plan for quality supervision spot checks on e-cigarette products sold in Shenzhen in the second half of 2024.
Sampling method
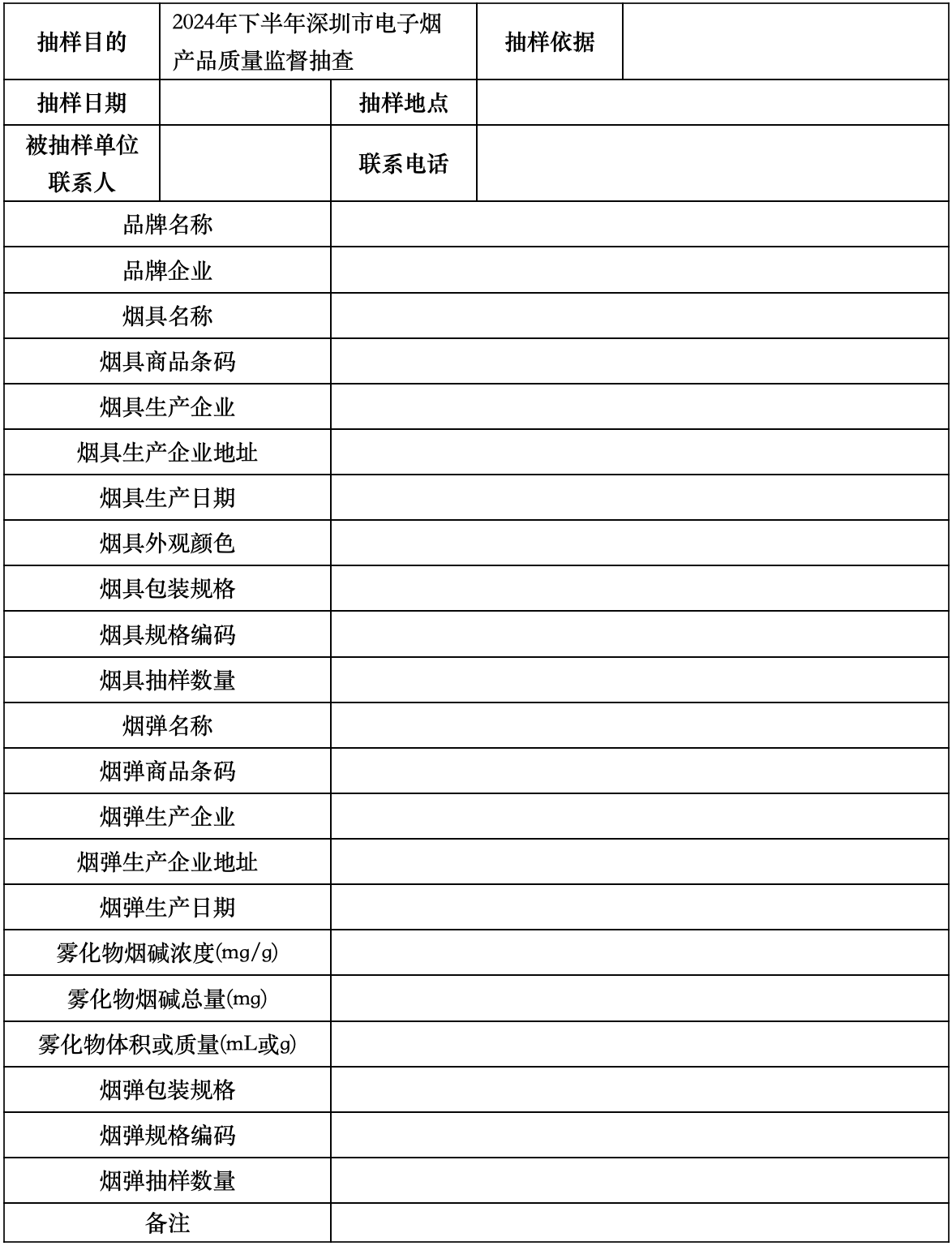
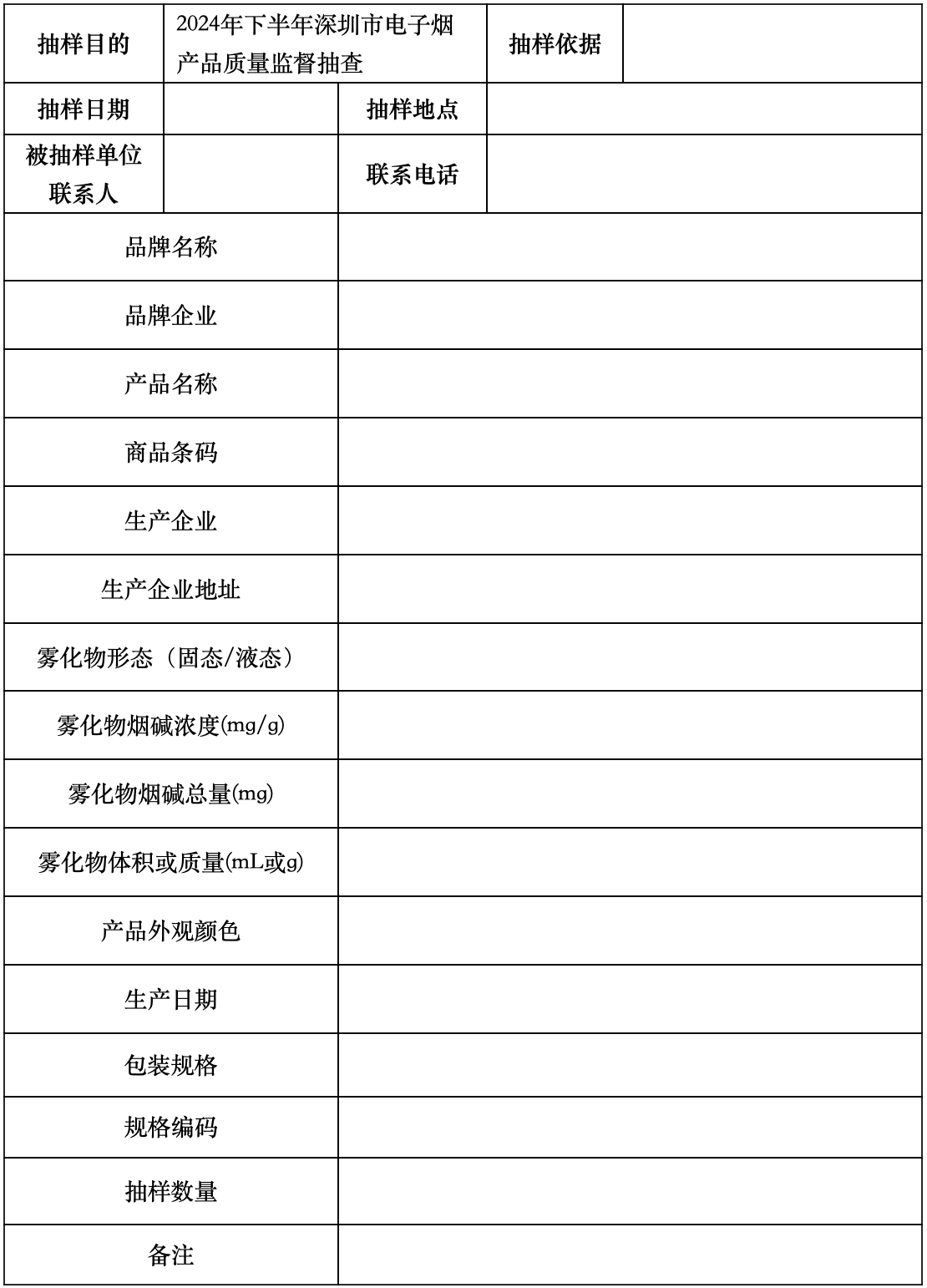
By randomly sampling, 75 smoking accessories and compatible pods (or combination products) of each type are selected, as well as at least 80 grams of aerosolized material, divided into test samples and backup samples as required. Test samples are sent to the relevant testing institution, while backup samples are temporarily stored at the institution responsible for sampling and used as needed for spot checks. The sampling form can be found in Appendix 1.
2. Examination criteria
Judgment Rules
Based on standards.
GB 41700 refers to an e-cigarette product.
3.2 Judgment principle
After conducting tests, it was found that all tested items met the standard requirements, determining the randomly sampled products to be compliant. If any item in the test does not meet the standard requirements, the randomly sampled product is considered non-compliant.
4. Conclusion
This scheme is applicable for quality supervision spot checks on e-cigarette products sold within Shenzhen city in the second half of 2024.
Appendix 1: Sample List of E-cigarette Products
Appendix 2: Method for detecting additives in e-cigarette aerosols.
Appendix 1
E-cigarette product sampling form (applicable to individual or combined sales of smoking products, pods)
Reference Number:
Sampling Unit (Official Seal): Sampled Unit (Official Seal):
Sample person: Investigator
E-cigarette Product Sampling Form (for disposable e-cigarette products)
Serial number:
Sampling unit (official stamp): Sampled unit (official stamp):
Sample person: Handler:
Appendix 2
Method for detecting additives in e-cigarette aerosols
E-cigarette aerosol additives determination of ester compounds and D-limonene by gas chromatography-mass spectrometry (Q/CTQTC122-2021).
E-cigarette aerosol additives determination of alcohol compounds by gas chromatography" (Q/CTQTC124-2021).
E-cigarette aerosol additive determination of catechins and epicatechins by spectrophotometry and high-performance liquid chromatography" (Q/CTQTC125-2021), this spot check only measures catechins.
E-cigarette vapor additives Determination of heterocyclic compounds Gas chromatography-mass spectrometry coupling method (Q/CTQTC121-2021).
E-cigarette aerosol additive determination of ketones by gas chromatography-mass spectrometry" (Q/CTQTC123-2021).
The determination of e-cigarette aerosol additives such as eugenol, maltol, ethyl maltol, vanillin, and ethyl vanillin by high-performance liquid chromatography (Q/CTQTC126-2021).
Title: Determination of Volatile Fatty Acids (Acetic Acid, Propionic Acid, Butyric Acid, and 2-Methylbutyric Acid) in E-Cigarette Aerosol Additives by Gas Chromatography-Mass Spectrometry Coupled Method (Q/CTQTC127-2021)
Analysis of Non-volatile Organic Acids in E-liquid Additives for E-cigarettes by Liquid Chromatography-Tandem Mass Spectrometry (Q/CTQTC128-2021)
Since the submission deadline for Pre-Market Tobacco Product Applications (PMTA) in September 2020, the agency has only authorized three types of electronic cigarette devices.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








