
On August 17th, Shanghai Shunho New Material Technology Co., Ltd. (Stock abbreviation: Shunho shares, stock code: 002565) released its semi-annual report for the year 2024.
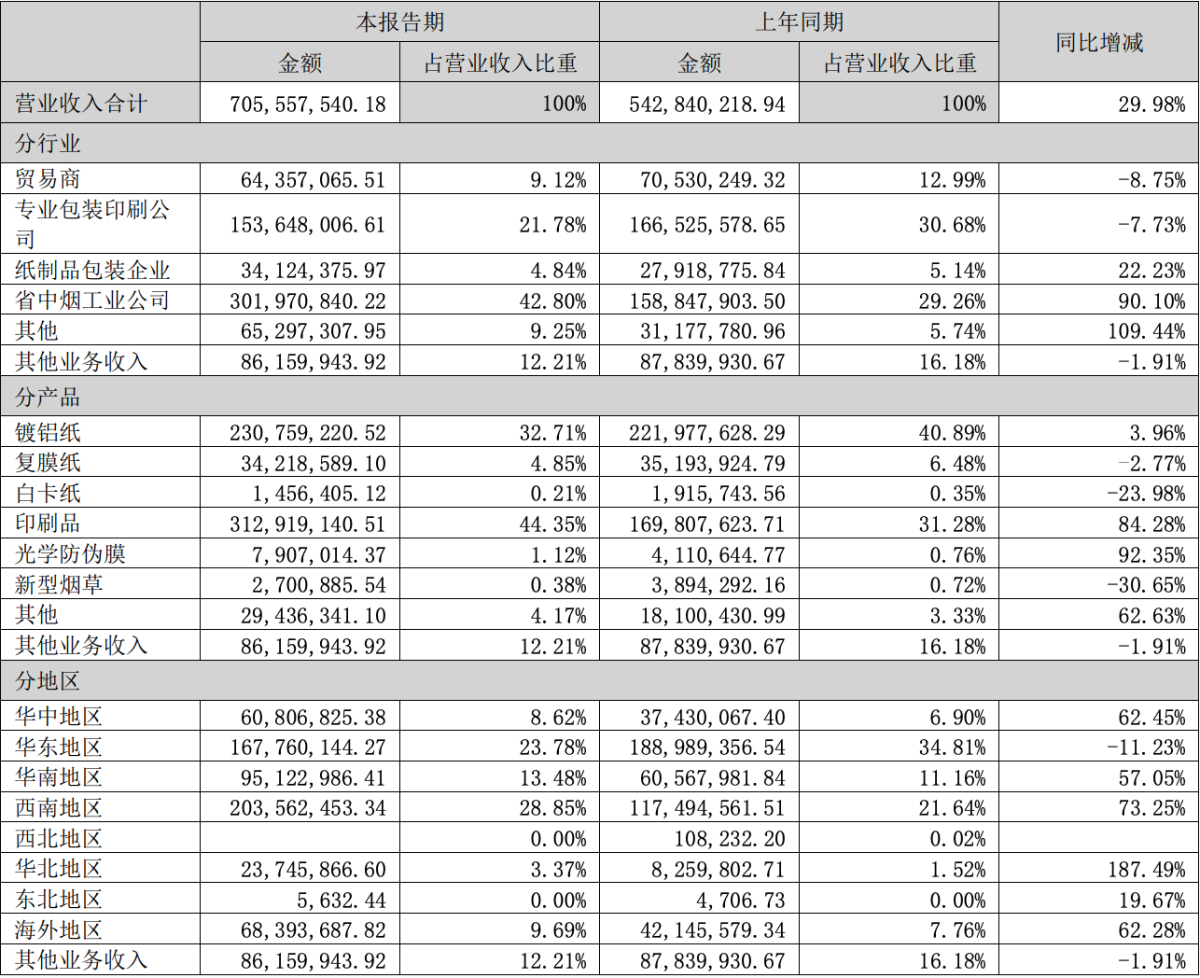
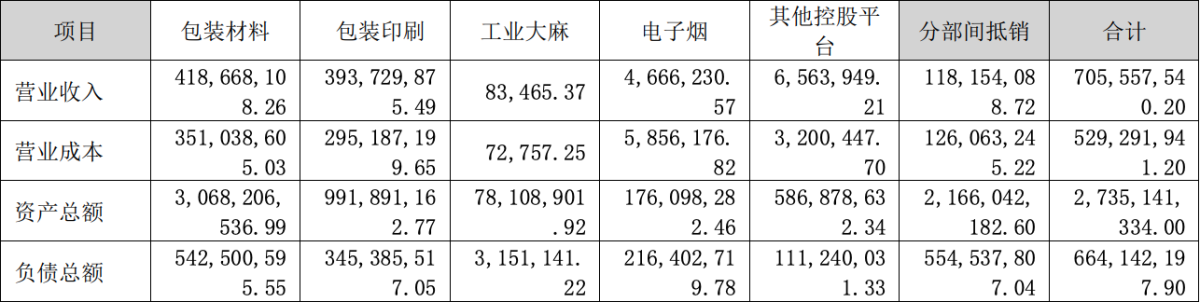
A report shows that the company achieved operating revenue of approximately 706 million yuan during the reporting period, an increase of 29.98% year-on-year. However, the company's business in the field of new tobacco products has decreased, with operating revenue of 2,700,885.54 yuan, accounting for 0.38% of the total operating revenue of the company, a decrease of 30.65% from the same period last year. This may be due to factors such as changes in market environment, policy adjustments, or company strategic realignments. The operating revenue from e-cigarette projects was 4.66 million yuan, while the industrial hemp project had operating revenue of 83,500 yuan.
According to reports, Shunho's subsidiary Greencore has obtained the Tobacco Monopoly Production Enterprise License for e-cigarette processing companies in the new tobacco field. Its invested company, Meizhonglian, has obtained the Tobacco Monopoly Production Enterprise License for e-cigarette product manufacturing companies. Meanwhile, Shunho's subsidiary Yilong has obtained the Tobacco Monopoly Production Enterprise License for e-cigarette brand holding companies for domestic sales.
The company stated that its subsidiary Shunho Yilong's "Yilong" brand e-cigarette has been launched in some retail stores in Beijing, Shenzhen, and Shanghai areas, and will continue to promote the listing of "Yilong" products in multiple locations in the future.
In addition, the company's wholly-owned subsidiary Yunnan Lvxin has obtained the "Yunnan Province Industrial Hemp Processing License," which means the company is qualified to process industrial hemp in Qujing City, Yunnan Province. To seize the rapidly growing opportunities in the overseas industrial hemp market, Shunho Corporation has established a subsidiary, Kinneloa Holdings Inc., in the United States. With legal qualifications to carry out industrial hemp processing and manufacturing related business in the US, as well as sales in other legal countries and regions worldwide. Kinneloa Holdings Inc.'s wholly-owned subsidiary, E1011 Labs, has launched ELON second-generation HNB devices and Stelo heating rod products.
Another wholly-owned subsidiary of the company, Vitaldiol Pharmaceutical, has launched the Vitaldiol – R Series (Recover, Relief, Rest) products and Essential elixirs for the US market. These products contain ingredients such as CBD, NMN (Nicotinamide Mononucleotide), turmeric, melatonin, and others, emphasizing a "minimalist ingredients" and "natural health" concept.
The report mentioned that the global e-cigarette market is expected to continue growing, and the company stated that they will increase research and development investment to further expand the new tobacco industry both domestically and overseas.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








