
On June 21st, the U.S. Food and Drug Administration (FDA) announced the approval of four menthol-flavored e-cigarette products from NJOY, marking the first time the FDA has allowed menthol-flavored e-cigarette products to be legally sold in the U.S. The approved products include two NJOY ACE products and two NJOY DAILY products.
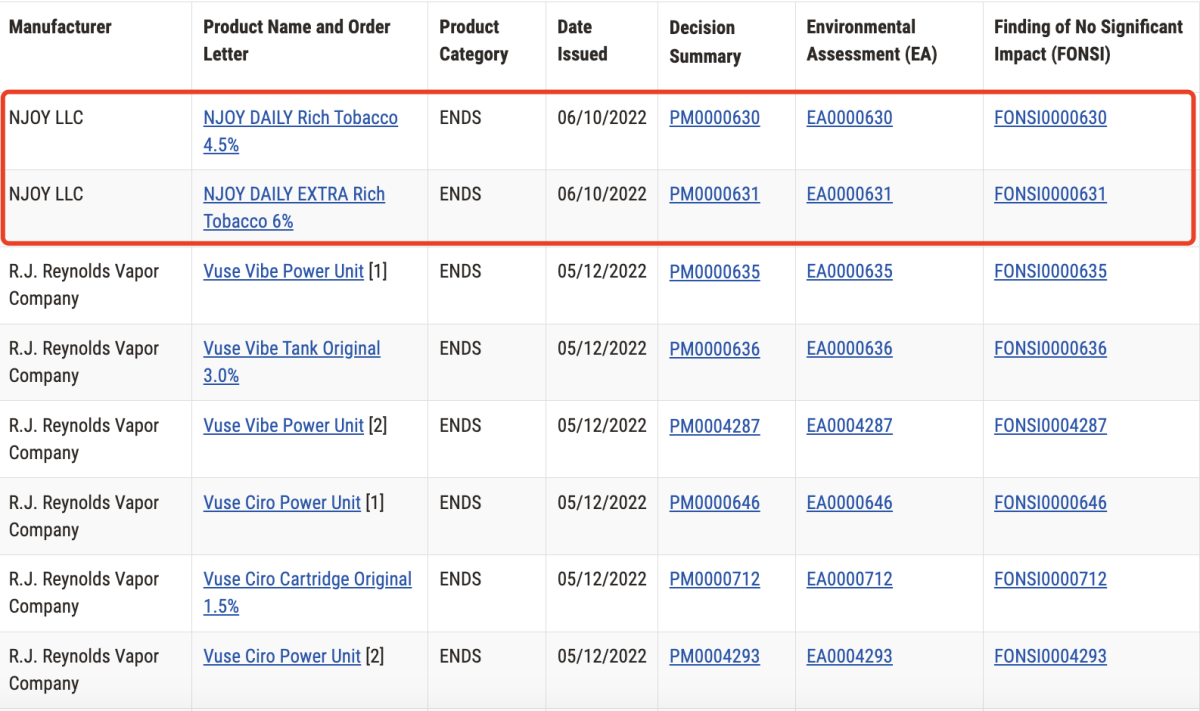
It has been nearly two years since the last time the FDA issued a marketing authorization order (MAO) for e-cigarettes. On June 10, 2022, the FDA approved NJOY's NJOY DAILY Rich Tobacco 4.5% and NJOY DAILY EXTRA Rich Tobacco 6% products through the premarket tobacco product application (PMTA) process.
Which E-cigarette will Come out on Top?
The FDA announced that it has approved a total of 27 e-cigarette products for sale. Among them, Japan Tobacco's Logic brand has 8 products, Altria's NJOY has 10 products, and British American Tobacco's Vuse has 9 products.
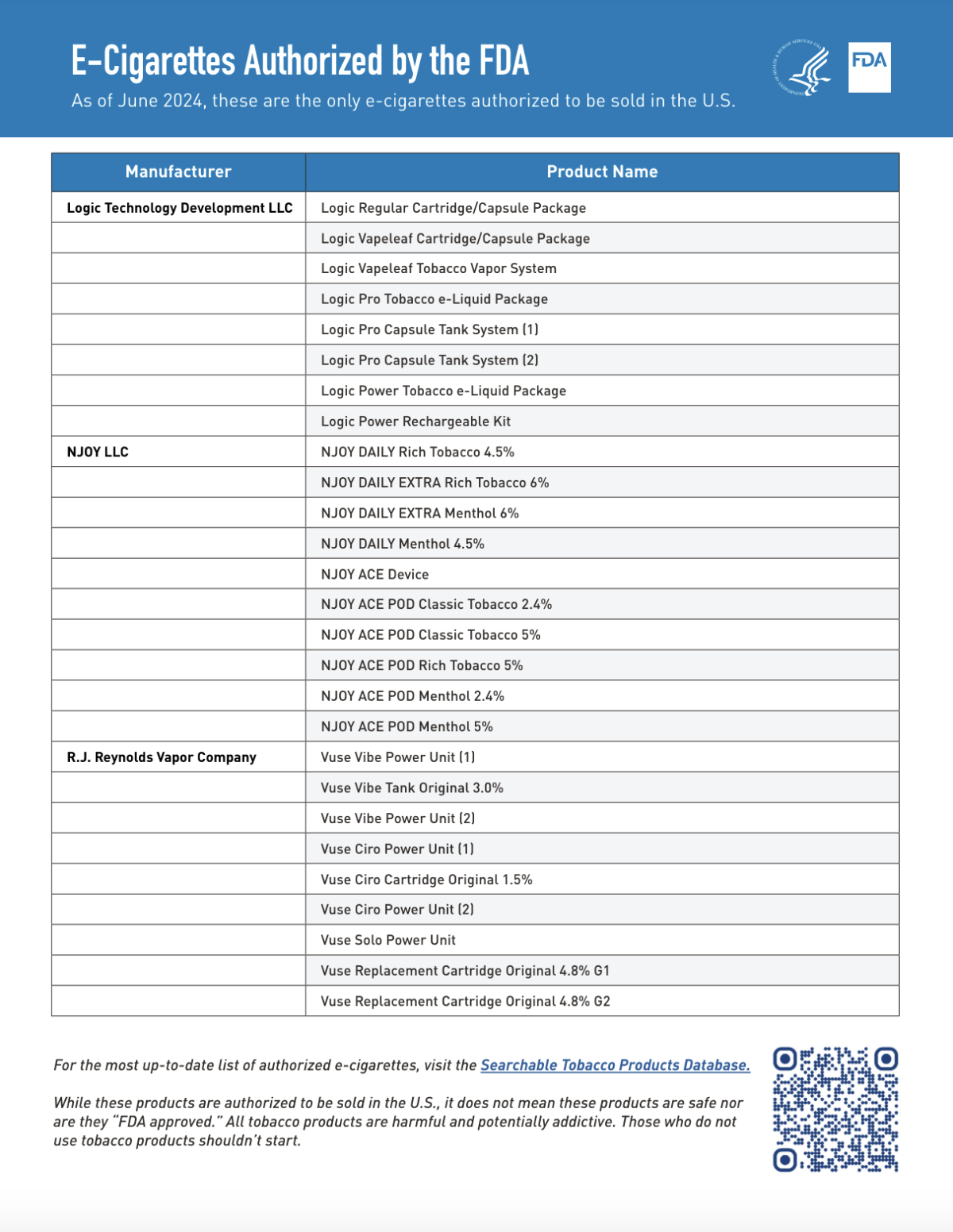
Among them, Logic LLC's e-cigarette products include:
- Logic Regular Cartridge/Capsule Package
- Logic Vapeleaf Cartridge/Capsule Package
- Logic Vapeleaf Tobacco Vapor System
- Logic Pro Tobacco e-Liquid Package
- Logic Pro Capsule Tank System (two models)
- Logic Power Tobacco e-Liquid Package
- Logic Power Rechargeable Kit
NJOY LLC's e-cigarette products include:
- The NJOY DAILY series includes various tobacco and menthol flavors in different strengths.
- The NJOY ACE Device;
- NJOY ACE POD series also offer different strengths of classic tobacco and menthol flavors.
R.J. Reynolds Vapor Company's e-cigarette products are part of this lineup:
- Vuse Vibe series, which includes the Power Unit and Tank
- Vuse Ciro series, which includes the Power Unit and Cartridge
- Vuse Solo Power Unit
- Vuse Replacement Cartridge, available in different versions of Original flavor.
How are the Product Specifications Approved for NJOY?
On the American e-cigarette website, 2FIRSTS found basic information on the four approved e-cigarettes, including two closed pod systems with menthol flavor (NJOY ACE Pod Menthol 2.4% and NJOY ACE Pod Menthol 5%) and two disposable menthol-flavored e-cigarettes (NJOY DAILY Menthol 4.5% and NJOY DAILY EXTRA Menthol 6%).
The NJOY ACE Pod Menthol 2.4%/5% includes two pods per package. This product offers two different nicotine concentration options, 24 mg/ml and 50 mg/ml, with each pod containing 1.9 ml of e-liquid made with nicotine salts and featuring a menthol flavor profile. The product is available on the e-cigarette retail website electrictobacconist for $9.99.

NJOY DAILY is a disposable e-cigarette with a nicotine concentration of 45 milligrams per gram (4.5% by weight), made with nicotine salts and flavored with menthol. On the other hand, NJOY DAILY Extra is similar to NJOY DAILY but with a higher nicotine concentration of 60 milligrams per gram.

Who Manufactures NJOY Products?
On June 24, Smoore International (06969.HK) issued a statement, which stated that the group's client NJOY LLC has received marketing authorization for ten new tobacco products through the pre-market tobacco application pathway. The statement also mentioned that "the group supplies these ten products to NJOY LLC." This indicates that Smoore International is one of the manufacturers of NJOY's compliant products.
According to an article on the official website of FEELM, a technology brand under Smoore, Smoore began its partnership with NJOY as early as 2009, and subsequently launched the NJOY King, Daily, and Ace series, quickly capturing market share after their release. On April 27, 2022, NJOY's product NJOY Ace officially passed the PMTA, making NJOY the first non-tobacco company to receive FDA approval.

It is worth noting that in June 2023, Ochiai officially acquired the full ownership of the third largest e-cigarette brand in the United States, NJOY, for a total of 2.75 billion US dollars. In the proposed acquisition agreement released in March of the same year, Ochiai directly included NJOY's technology and production partner, Smoore, in the announcement.
NJOY has a significant business partnership with Shenzhen Smoore Technology Limited (Smoore), a prominent company known for innovations in the e-cigarette industry, to create and produce its vaping products.

NJOY's Challenges and Responses
NJOY's menthol-flavored products receiving PMTA approval is undoubtedly a significant event in the development history of e-cigarettes in the United States. Compliance expert Zheng Zhi, in an interview with 2FIRSTS, stated that FDA's approval of menthol-flavored e-cigarettes is a positive signal, indicating a good development trend for flavored e-cigarettes in the United States. He expects that in the next 2-3 years, FDA will relax restrictions on the review of flavored products.
NJOY still has a limited market share in the current US market and consistently holds less than 5% of the market share. However, with the support of Achiria, NJOY seems to be showing signs of a "comeback".
After a year of continuous "infusions," according to the latest report from the American Nielsen convenience store (as of the four weeks ending June 1), NJOY's market share is 3.4%, up from 3.3% in the previous report.
It should be noted that among the main e-cigarette manufacturers in the United States, NJOY is the only company experiencing positive sales growth within the past four weeks, with an increase of 8%.
After abandoning JUUL, Altria is now focusing all its attention on developing NJOY's business, while its "interest" in flavored e-cigarettes continues to grow. According to a report by Bloomberg on February 21, Altria is in the final stages of confirming the application it will submit to the U.S. Food and Drug Administration to sell blueberry and watermelon flavored NJOY e-cigarette products. At the time, Altria CEO Billy Gifford stated that they were waiting for the FDA's response regarding menthol e-cigarettes.
Jaffrey Gothard also stated that Altria plans to introduce their regular tobacco-flavored NJOY e-cigarette products into 100,000 stores by 2024, as well as using new packaging. He estimates that the international sales opportunities for heating tobacco and e-cigarette products are worth between $35 billion and $50 billion.

In May 2024, the official website of Altria announced that NJOY had declared their new product, the NJOY ACE 2.0, would be submitting a PMTA to the FDA. This new device utilizes access restriction technology that can prevent minors from connecting via Bluetooth.
We are excited to build on our existing FDA authorized products with the NJOY ACE 2.0, which includes key technology features to prevent underage access to flavored NJOY products, while responsibly offering flavor options for adult smokers and e-cigarette enthusiasts," said Shannon Leistra, President and CEO of NJOY.
According to current publicly available information, NJOY ACE 2.0 is still under review by the FDA, with the review process expected to last several months. Analysts point out that if the product successfully passes FDA review, NJOY's position in the e-cigarette market will be further solidified, and they may be able to achieve Altria's vision in the emerging tobacco industry.
Scheme: Targeting Chinese Companies
In addition to expanding channels at the marketing level, Ochiai took action earlier in the legal aspect. In an announcement released by Ochiai in October 2023, NJOY announced a wide-ranging lawsuit against 34 domestic and foreign manufacturers, distributors, and online retailers, accusing them of illegal sales of disposable e-cigarette products, violating laws in California and other areas.
This lawsuit alleges that these companies are manufacturing, distributing, promoting, and selling products that violate California's taste ban laws, violate federal laws, and are sanctioned by the FDA, unfairly competing with companies that comply with state and federal laws.
This lawsuit was filed in the federal district court in California and involves four charges: unfair competition, false advertising, violations of the Lanham Act, and the Prevent All Cigarette Trafficking Act of 2009.
This list includes nearly all the mainstream e-cigarette brands in the American market at that time, including Breeze, Elf Bar, EBDesign, EB Create, Esco Bar, Flum, Juice Box, Lava Plus, Loon, Lost Mary, Mr. Fog, and Puff Bar. In addition to domestic American companies, the accused foreign companies are all based in China.
Confusion: Why NJOY?
2FIRSTS noticed that many media outlets referred to the news as "menthol-flavored e-cigarette," but in the official announcement by the US FDA, the term "menthol-flavored" was specifically used. In fact, there is a distinction between menthol-flavored and mint flavor.
- Menthol is an organic compound extracted from mint and other mint plants.
- The mint flavor is a more widely recognized taste that can come from menthol and other compounds such as menthone, menthol, and camphor.
Back in 2009, with the advent of the Tobacco Control Act, the FDA announced a ban on cigarette flavors, including mint, but not menthol. The rationale was that mint flavor is a type of tobacco flavor, while menthol is a natural extract of mint; menthol cigarettes provide a cooling sensation when used but do not offer any taste.
NJOY has now become the first brand of menthol e-cigarettes approved by the FDA, but it is not the first brand to attempt to pass the PMTA (Premarket Tobacco Product Application).
In the United States, menthol is extremely common in cigarettes, and tobacco companies that hold the market's voice have all tried to replicate the success of menthol cigarettes in their e-cigarette products.
However, whether it is JT's (Japan Tobacco) logic or BAT's (British American Tobacco) VUSE, all have failed.
In October 2022, the FDA issued a Marketing Denial Order (MDO) for several menthol e-cigarette products sold by Logic. These products include Logic Pro Menthol e-liquid packaging and Logic Power Menthol e-liquid packaging. This was the first time the FDA issued an MDO for menthol products after undergoing scientific review.
In March 2023, the FDA issued a sales ban (MDO) for two menthol e-cigarette products of the Vuse Solo brand under Reynolds American Company, including "Vuse Menthol 4.8% G1" and "Vuse Menthol 4.8% G2". The FDA stated that Reynolds American Company is not allowed to sell these products in the United States, otherwise, it will face FDA enforcement actions.
In October 2023, the FDA issued Marketing Denial Orders (MDOs) for six flavored e-cigarette products of the Vuse Alto brand, including three menthol flavors and three blueberry flavors.
In this announcement, the FDA stated that data from Altria shows that NJOY e-cigarettes can help smokers reduce exposure to harmful chemicals found in traditional cigarettes.
The FDA's review report still needs careful analysis. The Associated Press pointed out that the FDA is facing its own set court deadline to end the review of major e-cigarette brands such as JUUL and VUSE, which has been going on for years.
Previously, the FDA's review deadline has been postponed time and time again. The "covered application" refers to products that were on the market as of August 8, 2016, and were submitted to the FDA before September 9, 2020, covering products sold under brands such as JUUL, Vuse, NJOY, Logic, Blu, SMOK, Suorin, or Puff Bar.
Since the beginning of this year, the FDA has successively issued MDOs for products of brands such as SMOK, Bidi Vapor, Suorin, myblu, MNGO, etc.
According to the FDA's latest status report, as of March 31, the FDA has completed the review of 94% of the covered applications and is expected to take action on all remaining covered applications before June 30, 2024. The FDA stated that it would submit the next status report to the court before July 22.
Despite the FDA's strict and lengthy review process, many companies are still submitting PMTAs one after another. This is not only because the PMTA itself is a necessary condition for listing. In the view of senior PMTA certification consultant Zheng Zhi, international tobacco companies such as British American Tobacco, Altria, and JUUL insist on applying for PMTA. The intention is not only to promote specific products but also to "open the door," establish a close relationship with the FDA, build an approval process, accelerate the listing of their products, and enter the huge American market.
In the view of groups opposing e-cigarettes and tobacco, this decision by the FDA is "harming" teenagers and is contrary to the trend of regulation. Regulatory authorities have been urging tobacco products with menthol and other flavors that may attract teenagers to withdraw from the market for many years.
These organizations opposing e-cigarettes regard this action as a "Pandora's box," believing that the precedent set by the FDA will have a negative impact on teenagers:
"The FDA has once again disappointed American families because it allows predatory industries to acquire their next generation of lifelong customers - American children."
Although NJOY has won the "first" in history, British American Tobacco and Altria obviously have more concerns. They are urging the FDA to strengthen the enforcement of "illegal" synthetic nicotine e-cigarettes in the American e-cigarette market, as these illegal products are estimated to account for about half of the overall e-cigarette market share.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








